All AbMole products are for research use only, cannot be used for human consumption.
Cisplatin is an inorganic platinum complex that inhibits DNA synthesis by forming DNA crosslinking agents, inactivates GPXs, reduces cell GSH and induces iron death in HCT116 and A549 cells.
DMSO can inactivate Cisplatin's activity!

Kaohsiung J Med Sci. 2025 Jul 03; .
Rhaponitin Reverses Cisplatin Resistance and Impairs Cancer Stemness Through HIF‐1α/MCT4/Wnt Pathway in Tongue Squamous Cell Carcinoma
Cisplatin purchased from AbMole

bioRxiv. 2024 Jun 28;14(7):999.
Functionality of BRCA1 supports the survival of prostate cancer cells during the development of castration resistance
Cisplatin purchased from AbMole

Molecules. 2023 Mar 16;28(6):2685.
Chemical Constituents of Thesium chinense Turcz and Their In Vitro Antioxidant, Anti-Inflammatory and Cytotoxic Activities
Cisplatin purchased from AbMole

J Immunother Cancer. 2022 Oct;10(10):e005270.
CUL3/SPOP complex prevents immune escape and enhances chemotherapy sensitivity of ovarian cancer cells through degradation of PD-L1 protein
Cisplatin purchased from AbMole

Sci Rep. 2022 Oct 17;12(1):17358.
Screening of the siGPCR library in combination with cisplatin against lung cancers
Cisplatin purchased from AbMole

Phytochemistry. 2022 Dec 12;207:113558.
Diverse alkaloids from the aerial parts of Aconitum carmichaelii and antiproliferative activity of costemline via inhibiting SIRT1/ROCK1/P-STAT3 pathways
Cisplatin purchased from AbMole

Evid Based Complement Alternat Med. 2022 Apr 6;2022:4445293.
Zhenzhu Xiaoji Decoction Induces Autophagy and Apoptosis Cell Death in Liver Cancer Cells through AKT/mTOR and JAK2/STAT3 Signaling Pathway
Cisplatin purchased from AbMole

Clin Epigenetics. 2021 Jul 22;13(1):142.
Genome-wide DNA methylome analysis identifies methylation signatures associated with survival and drug resistance of ovarian cancers
Cisplatin purchased from AbMole

Bioengineered. 2021 Dec;12(1):2499-2510.
Targeting the miR-6734-3p/ZEB2 axis hampers development of non-small cell lung cancer (NSCLC) and increases susceptibility of cancer cells to cisplatin treatment
Cisplatin purchased from AbMole

Aging (Albany NY). 2020 Jun 9;12(11):11025-11041.
LncRNA ADAMTS9-AS2 inhibits gastric cancer (GC) development and sensitizes chemoresistant GC cells to cisplatin by regulating miR-223-3p/NLRP3 axis
Cisplatin purchased from AbMole

Open Life Sciences. 2020 June;15(1):409-417.
Buformin suppresses osteosarcoma via targeting AMPK signaling pathway
Cisplatin purchased from AbMole

2020 Dec.
A Novel Circular RNA hsa_circRNA_103809/miR-377-3p/GOT1 Pathway Regulates Cisplatin-Resistance in Non-Small Cell Lung Cancer (NSCLC)
Cisplatin purchased from AbMole
Cell Cycle. 2018;17(10):1235-1244.
Apatinib, a novel tyrosine kinase inhibitor, suppresses tumor growth in cervical cancer and synergizes with Paclitaxel.
Cisplatin purchased from AbMole
Cancer Biol Ther. 2018 Feb 5.
Buformin suppresses proliferation and invasion via AMPK/S6 pathway in cervical cancer and synergizes with paclitaxel
Cisplatin purchased from AbMole

J Korean Med Sci. 2016 Jun;31(6): 836–842.
Silencing of ABCG2 by MicroRNA-3163 Inhibits Multidrug Resistance in Retinoblastoma Cancer Stem Cells
Cisplatin purchased from AbMole
| Cell Experiment | |
|---|---|
| Cell lines | A549-luc cell |
| Preparation method | Cells were allowed to adhere for 24 hours and subsequently incubated with either PRINT-Platin or cisplatin at concentrations ranging from 9.8 nM to 80 µM (drug concentration) for 72 hours at 37 °C in a humidified 5 % CO2 atmosphere. After the incubation period, all media/particles were aspirated off cells. 100 µL fresh medium was added back to cells, followed by the addition of 100 µL CellTiter-Glo® Luminescent Cell Viability Assay reagent. |
| Concentrations | 9.8 nM to 80 µM |
| Incubation time | 72 h |
| Animal Experiment | |
|---|---|
| Animal models | healthy mice |
| Formulation | pH-adjusted 0.9 wt% NaCl solution |
| Dosages | 3 mg/kg |
| Administration | vein injection |
| Molecular Weight | 300.05 |
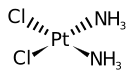
| Formula | Cl2H6N2Pt |
| CAS Number | 15663-27-1 |
| Solubility (25°C) | DMF: 9 mg/mL (Need ultrasonic) Water ~1 mg/mL (ultrasonic and heating) |
| Storage | 2-8°C, protect from light |
| Related DNA/RNA Synthesis Products |
|---|
| AV-153 free base
AV-153 free base is an antimutagenic. AV-153 free base intercalates to DNA in a single strand break and reduces DNA damage, stimulates DNA repair in human cells in vitro. AV-153 free base interacts with thymine and cytosine and has an influence on poly(ADP)ribosylation. |
| Deoxyribonucleic Acid (from Salmon sperm)
Deoxyribonucleic Acid (from Salmon sperm) can be used as a research reagent, widely used in molecular biology, pharmacology and other scientific research. |
| GSK4418959
GSK4418959 (IDE275) is a non-covalent, reversible, selective and orally active WRN helicase inhibitor. GSK4418959 inhibits ATPase and DNA unwinding functions in an ATP-competitive manner. |
| Dencatistat
Dencatistat (P115) is an inhibitor of Cytidine Triphosphate Synthase 1 (CTPS1) with an IC50 value of ≤ 0.1 μM. |
| Antipain dihydrochloride
Antipain dihydrochloride is a protease inhibitor. Antipain dihydrochloride inhibits N-methyl-N'-nitro-N-nitrosoguanidine (MNNG)-induced transformation and increases chromosomal aberrations. Antipain dihydrochloride also restricts uterine DNA synthesis and function in mice. |
All AbMole products are for research use only, cannot be used for human consumption or veterinary use. We do not provide products or services to individuals. Please comply with the intended use and do not use AbMole products for any other purpose.


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2024 AbMole BioScience. All Rights Reserved.
