All AbMole products are for research use only, cannot be used for human consumption.
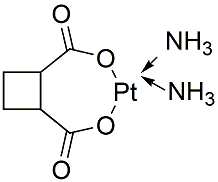
Carboplatin (NSC 241240) is a second-generation platinum compound with a broad spectrum of antineoplastic properties. Carboplatin is activated intracellularly to form reactive platinum complexes that bind to nucleophilic groups such as GC-rich sites in DNA, thereby inducing intrastrand and interstrand DNA cross-links, as well as DNA-protein cross-links. Carboplatin possesses tumoricidal activity similar to that of its parent compound, cisplatin, but is more stable and less toxic. Carboplatin is used against some forms of cancer (mainly ovarian carcinoma, lung, head and neck cancers).
DMSO can inactivate Carboplatin's activity

Int J Mol Sci. 2022 Jun 3;23(11):6290.
Carboplatin-Induced Thrombocytopenia through JAK2 Downregulation, S-Phase Cell Cycle Arrest, and Apoptosis in Megakaryocytes
Carboplatin purchased from AbMole

Int J Oncol. 2020 Jan;56(1):47-68.
Circulating non‑coding RNA‑biomarker potential in neoadjuvant chemotherapy of triple negative breast cancer?
Carboplatin purchased from AbMole

Universitat Freiburg im Breisgau. 2020 Jul.
Investigation of chemotherapy-induced non-coding RNA expression alterations in clinical breast cancer management
Carboplatin purchased from AbMole

Cell Death Dis. 2018 Feb 12;9(2):213.
Autophagy mediates glucose starvationinduced glioblastoma cell quiescence and chemoresistance through coordinating cell metabolism, cell cycle, and survival
Carboplatin purchased from AbMole
Cell Cycle. 2018;17(10):1235-1244.
Apatinib, a novel tyrosine kinase inhibitor, suppresses tumor growth in cervical cancer and synergizes with Paclitaxel.
Carboplatin purchased from AbMole
| Cell Experiment | |
|---|---|
| Cell lines | 5637 cells |
| Preparation method | Cells were dosed and incubated with 290 μM carboplatin or 290 μM carboplatin combined with 0.08 μM paclitaxel for 4 h, each including 0.43 μM[14C]carboplatin (106 dpm/ml). After washing, cells were maintained with 14C carboplatin-free culture media. DNA was extracted from cells and converted to graphite and measured by AMS for 14C quantification as previously described. |
| Concentrations | 290 μM |
| Incubation time | 4 h |
| Animal Experiment | |
|---|---|
| Animal models | Female 6‐week‐old nude mice |
| Formulation | |
| Dosages | 100 mg/kg |
| Administration | s.c. |
| Molecular Weight | 371.25 |
| Formula | C6H12N2O4Pt |
| CAS Number | 41575-94-4 |
| Solubility (25°C) | Water 4 mg/mL warmed DMF 1 mg/mL (Need ultrasonic) |
| Storage | 4°C, protect from light, sealed |
| Related DNA/RNA Synthesis Products |
|---|
| Deoxyribonucleic Acid (from Salmon sperm)
Deoxyribonucleic Acid (from Salmon sperm) can be used as a research reagent, widely used in molecular biology, pharmacology and other scientific research. |
| GSK4418959
GSK4418959 (IDE275) is a non-covalent, reversible, selective and orally active WRN helicase inhibitor. GSK4418959 inhibits ATPase and DNA unwinding functions in an ATP-competitive manner. |
| Dencatistat
Dencatistat (P115) is an inhibitor of Cytidine Triphosphate Synthase 1 (CTPS1) with an IC50 value of ≤ 0.1 μM. |
| Antipain dihydrochloride
Antipain dihydrochloride is a protease inhibitor. Antipain dihydrochloride inhibits N-methyl-N'-nitro-N-nitrosoguanidine (MNNG)-induced transformation and increases chromosomal aberrations. Antipain dihydrochloride also restricts uterine DNA synthesis and function in mice. |
| Tetrahydrouridine
Tetrahydrouridine (THU) is potent inhibitor of cytidine deaminase (CDA), which competitively blocks the enzyme's active site more effectively than intrinsic cytidine. |
All AbMole products are for research use only, cannot be used for human consumption or veterinary use. We do not provide products or services to individuals. Please comply with the intended use and do not use AbMole products for any other purpose.


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2024 AbMole BioScience. All Rights Reserved.
