All AbMole products are for research use only, cannot be used for human consumption.
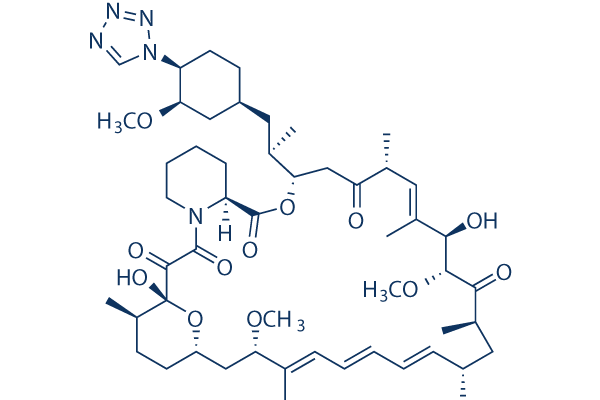
In vitro: Zotarolimus (ABT-578) is a semi-synthetic analogue of rapamycin, made by substituting a tetrazole ring for the native hydroxyl group at position 42 in rapamycin. Zotarolimus is highly effective in inhibiting both smooth muscle cell and endothelial cell proliferation, with IC50 values of 2.9 nM and 2.6 nM, respectively. Zotarolimus is mechanistically similar to sirolimus in having high-affinity binding to the immunophilin FKBP12 and comparable potency for inhibiting in vitro proliferation of both human and rat T cells. Zotarolimus inhibits Con A-induced human T cells and rat T cells proliferation with IC50 of 7.0 nM and 1337 nM respectively. In vivo: Zotarolimus-eluting stents effectively reduce neointima formation in a 28-day, well-characterized swine model of coronary artery restenosis. Zotarolimus appears effective in preventing neointimal thickening, reducing late loss from 1.03 to 0.62 mm with a 47% reduction in TVF compared with bare metal stents (15.4% with the Driver stent to 8.1% with the Endeavor stent). Zotarolimus is efficacious in suppressing adjuvant DTH, EAE, and cardiac allograft rejection with ED50 values of 1.72, 1.17, and 3.71 mg/kg/day, respectively.
| Cell Experiment | |
|---|---|
| Cell lines | Human coronary artery cells |
| Preparation method | Cell proliferation is assayed by measuring tritiated thymidine incorporation in vitro. Human coronary artery cells (hCa) are seeded into tissue culture flasks for expansion and applied to 96-well plates at desired density in complete media (5000 hCaSMC; 10 000 hCaEC). After 2 days, complete media is replaced with incomplete media to synchronize cells and induce G0 state. Two days later, incomplete media are removed and replaced with complete media (serum/growth factors) to induce G0 to G1 transition. Complete media also contain drug at desired concentrations to determine its effects on cell proliferation. On day 7, 3H-thymidine is added to cells to monitor DNA synthesis, and cells are harvested after overnight incorporation of radioactivity. After an incubation period of 72 h, 25 μL (1 μCi/well) of 3H-thymidine are added to each well. The cells are incubated at 37°C for 16-18 h to allow for incorporation of 3H-thymidine into newly synthesized DNA and the cells harvested onto 96-well plates containing bonded glass fibre filters . The filter plates are air-dried overnight, MicroScint-20 (25 μL) added to each filter well and counted. Drug activity is determined by the inhibition of 3H-thymidine incorporation into newly synthesized DNA relative to cells grown in complete media. |
| Concentrations | ~1 μM |
| Incubation time | 5 days |
| Animal Experiment | |
|---|---|
| Animal models | Male Sprague-Dawley rats |
| Formulation | ethanol: propylene glycol: cremophor EL: D5W vehicle (20: 30: 2: 48, by volume) |
| Dosages | 2.5 mg/kg |
| Administration | intravenous or oral |
| Molecular Weight | 966.21 |
| Formula | C52H79N5O12 |
| CAS Number | 221877-54-9 |
| Solubility (25°C) | 100 mg/mL in DMSO |
| Storage |
Powder -20°C 3 years ; 4°C 2 years In solvent -80°C 6 months ; -20°C 1 month |
| Related mTOR Products |
|---|
| RMC-4627
RMC-4627 is a selective mTORC1 inhibitor that activates 4EBP1 and inhibits tumor growth. |
| RMC-4529
RMC-4529 has an IC50 value of 1.0 nM against p-4E-BP1-(T37/46) in mTOR kinase cellular assay. |
| WYE-687 dihydrochloride
WYE-687 dihydrochloride is an ATP-competitive mTOR inhibitor with an IC50 of 7 nM. |
| Rapamycin-d3
Rapamycin-d3 |
| L-Leucine-d10
L-Leucine-d10 |
All AbMole products are for research use only, cannot be used for human consumption or veterinary use. We do not provide products or services to individuals. Please comply with the intended use and do not use AbMole products for any other purpose.


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2024 AbMole BioScience. All Rights Reserved.
