All AbMole products are for research use only, cannot be used for human consumption.
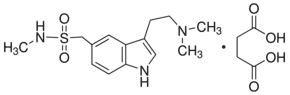
Sumatriptan succinate is a 5-HT1 serotonin receptor agonist. Sumatriptan reduces the vascular inflammation associated with migraine. Sumatriptan displays the highest affinity for 5-HT1D (Ki = 17 nM) and 5-HT1B (Ki = 27 nM) binding sites and is slightly less potent at 5-HT1A binding sites (Ki = 100 nM). Sumatriptan at a clinically relevant dose (100 mg/kg, s.c.) leads to a significant reduction of the mechanical allodynia-like behaviour on both the injured and the contralateral sides (peak-effects 6.3 g and 4.4 g, respectively) in a rat model of trigeminal neuropathic pain. Sumatriptan results in oral bioavailabilities of 37, 58 and 23% in rat, dog and rabbit, respectively. sumatriptan is cleared rapidly by metabolic and renal clearance with a half-life of 1-2 hour.

Cephalalgia. 2025 Feb 17;45(2):3331024251317446.
Chronification of migraine sensitizes to CGRP in male and female mice
Sumatriptan succinate purchased from AbMole

Cephalalgia. 2021 Sep 12;3331024211039813.
A prolactin-dependent sexually dimorphic mechanism of migraine chronification
Sumatriptan succinate purchased from AbMole

Cephalalgia. 2020 Aug;40(9):903-912.
Evaluation of LY573144 (lasmiditan) in a preclinical model of medication overuse headache
Sumatriptan succinate purchased from AbMole

Cephalalgia. 2020 Aug;40(9):892-902.
Ubrogepant does not induce latent sensitization in a preclinical model of medication overuse headache
Sumatriptan succinate purchased from AbMole

eNeuro. 2018 July 5;5(4):ENEURO.0116-18.
Loss of Blood-Brain Barrier Integrity in a KCl-Induced Model of Episodic Headache Enhances CNS Drug Delivery
Sumatriptan succinate purchased from AbMole

Cephalalgia. 2017 Jul;37(8):780-794.
Kappa opioid receptor antagonists: A possible new class of therapeutics for migraine prevention
Sumatriptan succinate purchased from AbMole
Cephalalgia. 2016 May 19.
Prevention of stress- or nitric oxide donor-induced medication overuse headache by a calcitonin gene-related peptide antibody in rodents.
Sumatriptan succinate purchased from AbMole
| Cell Experiment | |
|---|---|
| Cell lines | |
| Preparation method | |
| Concentrations | |
| Incubation time | |
| Animal Experiment | |
|---|---|
| Animal models | Adult male Sprague–Dawley rats model |
| Formulation | sterile 0.9% saline solution |
| Dosages | 0.6 mg/kg/day for 7 days |
| Administration | subcutaneously injection |
| Molecular Weight | 413.49 |
| Formula | C14H21N3O2S.C4H6O4 |
| CAS Number | 103628-48-4 |
| Solubility (25°C) | DMSO 50 mg/mL Water 90 mg/mL |
| Storage | -20°C, dry, sealed |
| Related 5-HT Receptor Products |
|---|
| PF-03382792
PF-03382792 is a potent 5-HT4 partial agonist with a Ki of 2.7 nM and an EC50 of 0.9 nM for 5-HT4d. PF-03382792 can penetrate the brain. |
| Tegaserod
Tegaserod is an orally active serotonin receptor 4 (5-HT4R) agonist and a 5-HT2B receptor antagonist. Tegaserod has pKis of 7.5, 8.4 and 7.0 for human recombinant 5-HT2A, 5-HT2B and 5-HT2C receptors, respectively. Tegaserod causes tumor cell apoptosis, blunts PI3K/Akt/mTOR signaling and decreases S6 phosphorylation. |
| cis-Urocanic acid
cis-Urocanic acid is a 5-HT2A receptor agonist. cis-Urocanic acid binds to 5-HT receptor with relatively high affinity (Kd=4.6 nM). cis-Urocanic acid is also an immune modulator that induces immunosuppression. |
| 5-HT2B antagonist-1
5-HT2B antagonist-1 is an orally active 5-HT2B receptor antagonist with an IC50 value of 33.4 nM. |
| AS19
AS19 is a potent, selective 5-HT7 receptor agonist with an IC50 value of 0.83 nM and a Ki of 0.6 nM. |
All AbMole products are for research use only, cannot be used for human consumption or veterinary use. We do not provide products or services to individuals. Please comply with the intended use and do not use AbMole products for any other purpose.


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2024 AbMole BioScience. All Rights Reserved.
