All AbMole products are for research use only, cannot be used for human consumption.
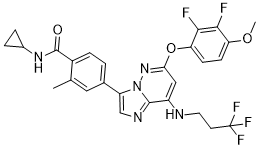
BAY 1217389 selectively binds to and inhibits the activity of Mps1. This inactivates the spindle assembly checkpoint (SAC), accelerates mitosis, causes chromosomal misalignment and missegregation, and mitotic checkpoint complex destabilization. This induces cell death in Mps1-overexpressing cancer cells. BAY 1217389 efficiently inhibits tumor cell proliferation in vitro.
In vivo: BAY 1217389 achieves moderate efficacy in monotherapy in tumor xenograft studies. However, in line with its unique mode of action, when combines with paclitaxel, low doses of Mps1 inhibitor reduces paclitaxel-induced mitotic arrest in line with weakening of SAC activity. As a result, combination therapy strongly improves efficacy over paclitaxel or Mps1 inhibitor monotreatment at the respective MTDs in a broad range of xenograft models including those showing acquired or intrinsic paclitaxel-resistance. BAY 1217389 shows good tolerability without adding toxicity to paclitaxel monotherapy.
| Cell Experiment | |
|---|---|
| Cell lines | Tumor cell lines HeLa-MaTu and HeLa-MaTu-ADR cells |
| Preparation method | Cells were seeded into 96-well plates at densities ranging from 1,000 to 5,000 cells per well in the appropriate medium supplemented with 10% FCS. After 24 hours, cells were treated in quadruplicates with serial dilutions of compounds. After further 96 hours, adherent cells were fixed with glutaraldehyde and stained with crystal violet. IC50 values were calculated by means of a 4-parameter fit using the company's own software. |
| Concentrations | |
| Incubation time | 96 h |
| Animal Experiment | |
|---|---|
| Animal models | Male Wistar rats and female CD1 or NMRI nu/nu mice |
| Formulation | 50% PEG 400, 10% ethanol, and 40% water |
| Dosages | 1, 2, 4, or 8 mg/kg (p.o.) |
| Administration | i.v. or p.o. |
| Molecular Weight | 561.5 |
| Formula | C27H24F5N5O3 |
| CAS Number | 1554458-53-5 |
| Solubility (25°C) | DMSO: ≥ 60 mg/mL |
| Storage |
Powder -20°C 3 years ; 4°C 2 years In solvent -80°C 6 months ; -20°C 1 month |
[1] Wengner AM, et al. Mol Cancer Ther. Novel Mps1 Kinase Inhibitors with Potent Antitumor Activity.
| Related Kinesin Products |
|---|
| AZD4877
AZD4877 is another isostere to Ispinesiband also a kinesin spindle protein (Eg5) inhibitor with IC50 of 2 nM.AZD4877 arrests cell mitosis, leads to the formation of the monopolar spindle phenotype and induces apoptosis. |
| Filanesib TFA
Filanesib TFA (ARRY-520 TFA) is a selective kinesin spindle protein (KSP) inhibitor, with an IC50 of 6 nM for human KSP. |
| Filanesib hydrochloride
Filanesib (ARRY-520) hydrochloride is a selective and noncompetitive kinesin spindle protein (KSP) inhibitor, with an IC50 of 6 nM for human KSP. Filanesib induces cell death by apoptosis in vitro. |
| Eg5-IN-1
Eg5-IN-1 is a potent kinesin family motor protein (Eg5) inhibitor with an IC50 value of 1.97 µM. |
| PVZB-1194
PVZB-1194 is a biphenyl-type inhibitor of kinesin Eg5 that induces apoptosis by inhibiting Eg5 ATPase activity and shows potent kinesin spindle protein (KSP) inhibition only in the presence of microtubules, with anticancer activity. |
All AbMole products are for research use only, cannot be used for human consumption or veterinary use. We do not provide products or services to individuals. Please comply with the intended use and do not use AbMole products for any other purpose.


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2024 AbMole BioScience. All Rights Reserved.
