All AbMole products are for research use only, cannot be used for human consumption.
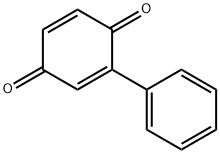
Phenyl-p-benzoquinone (PBQ; 2-Phenyl-1,4-benzoquinone) stimulus trigger a different peripheral cytokine cascade compared to acetic acid, it induces writhing response dependent on IL-18, IFNγ and endothelin-1.
| Molecular Weight | 184.19 |
| Formula | C12H8O2 |
| CAS Number | 363-03-1 |
| Solubility (25°C) | DMSO |
| Storage | 2-8°C, dry, sealed |
[5] Michael Emmanuel Mutseyekwa, et al. Water Sci Technol. Ozonation for the removal of bisphenol A
[9] J Esteve, et al. Gen Pharmacol. Pharmacological profile of droxicam
| Related Products |
|---|
| Myoglobin (from equine skeletal muscle)
Myoglobin is a small molecular pigment protein formed by binding globin to Heme, which can be reversibly bound to oxygen to form MbO2, MbO2 is called oxymyoglobin, and Mb is called deoxymyoglobin. Myoglobin has the role of transporting and storing oxygen in muscle cells. |
| Hemoglobin (from bovine blood)
Hemoglobin is a iron-containing protein in red blood cells with oxygen binding properties. Hemoglobin is an inducer of HO-1. Hemoglobin consits of heme, which binds to oxygen. Hemoglobin also transports other gases, such as carbon dioxide, nitric oxide, hydrogen sulfide and sulfide. |
| Diethylenetriaminepentaacetic dianhydride
Diethylenetriaminepentaacetic dianhydride (DTPA anhydride) is a bifunctional chelator whose anhydride can react with amino groups in proteins (such as lysine residues) to form stable amide bonds. Diethylenetriaminepentaacetic dianhydride (DTPA anhydride) can also bind to radionuclides to synthesize radionuclide-labeled drug conjugates (RDCs). |
| 3-Phenylthiophene
3-Phenylthiophene is a biochemical material that can be used in scientific research. 3-Phenylthiophene is a conducting polymer precursor. |
| DSPE-PEG-FA
DSPE-PEG2K-FA is a PEG derivative containing folic acid. DSPE-PEG2K-FA has a targeting effect and can bind to folic acid receptors in cancer cells. DSPE-PEG2K-FA forms micelles/lipid bilayers and can be used in research on targeted drug delivery systems. |
All AbMole products are for research use only, cannot be used for human consumption or veterinary use. We do not provide products or services to individuals. Please comply with the intended use and do not use AbMole products for any other purpose.


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2024 AbMole BioScience. All Rights Reserved.
