All AbMole products are for research use only, cannot be used for human consumption.
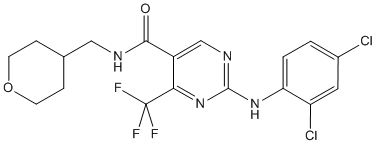
GW842166X shows similar potency and efficacy for rat and human recombinant CB2 receptors with EC50 of 91 nM and 63nM, respectively. GW842166X exhibits full agonist potency with an EC50 of 133 nM and Emax of 101% in cyclase assays. GW842166X exhibits weak agonist potency with an EC50 of 7.780 μM and Emax of 84% in FLIPR assays.GW842166X has an oral bioavailability of 58% and a half-life of 3 h when dosed orally in the rat. GW842166X has extremely high potency with an oral ED50 of 0.1 mg/kg and shows full reversal of hyperalgesia at 0.3 mg/kg in the FCAa model of inflammatory pain. GW842166X orally administrated at a dose of 15 mg/kg for 8 days produced a significant reversal of the CCI induced decrease in paw withdrawal threshold in a rat model of neuropathic pain.
| Cell Experiment | |
|---|---|
| Cell lines | |
| Preparation method | |
| Concentrations | |
| Incubation time | |
| Animal Experiment | |
|---|---|
| Animal models | rat model of neuropathic pain |
| Formulation | saline |
| Dosages | 15 mg/kg |
| Administration | Orally administrated once daily for 8 days |
| Molecular Weight | 449.25 |
| Formula | C18H17Cl2F3N4O2 |
| CAS Number | 666260-75-9 |
| Solubility (25°C) | DMSO 10 mg/mL |
| Storage |
Powder -20°C 3 years ; 4°C 2 years In solvent -80°C 6 months ; -20°C 1 month |
| Related Cannabinoid Products |
|---|
| UCM707
UCM707, a potent and selective inhibitor of endocannabinoid uptake, potentiates hypokinetic and antinociceptive effects of Anandamide. |
| SCH 336
SCH 336 is a potent, selective, inverse and orally active CB2 agonist. |
| JTE-907
JTE-907 is a highly selective, orally active CB2 receptor inverse agonist and exerts anti-inflammatory effects in vivo. |
| LEI-101
LEI-101 is a potent, selective, and orally bioavailable cannabinoid CB2 receptor agonist, with a pEC50 of 8 for hCB2, and a pKi of less than 4 for hERG. |
| AEF0117
AEF0117 is a signaling-specific inhibitor of the cannabinoid receptor 1 (CB1-SSi). |
All AbMole products are for research use only, cannot be used for human consumption or veterinary use. We do not provide products or services to individuals. Please comply with the intended use and do not use AbMole products for any other purpose.


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2024 AbMole BioScience. All Rights Reserved.
