All AbMole products are for research use only, cannot be used for human consumption.
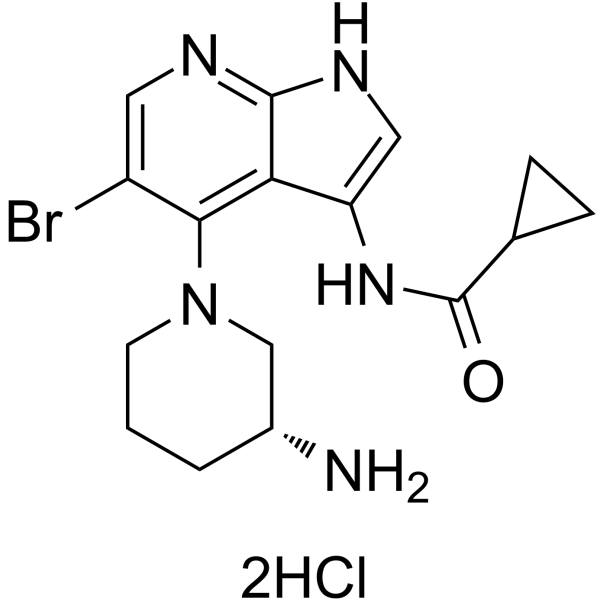
GDC-0575 dihydrochloride (ARRY-575 dihydrochloride) is an orally bioavailable CHK1 inhibitor, with an IC50 of 1.2 nM, and has antitumor activity.
| Molecular Weight | 451.19 |
| CAS Number | 1657014-42-0 |
| Solubility (25°C) | DMSO 65 mg/mL Water 25 mg/mL |
| Storage | 2-8°C, dry, sealed |
| Related Checkpoint Products |
|---|
| PV-1019
PV-1019 (NSC 744039) is a potent, selective Chk2 inhibitor with an IC50 value of 24 nM. PV-1019 inhibits IR-induced apoptosis. |
| CCT241533 dihydrochloride
CCT241533 dihydrochloride is a potent and selective ATP competitive inhibitor of CHK2 with an IC50 of 3 nM and Ki of 1.16 nM. |
| CBP501 Affinity Peptide
CBP501 Affinity Peptide is a Chk kinase inhibitor that can abrogate G2 arrest induced by DNA-damaging agents. |
| Chktide
Chktide is a substrate for CHK1 and CHK2. |
| Zn-DPA-maytansinoid conjugate 1
Zn-DPA-maytansinoid conjugate 1 is a small molecule-based maytansinoid conjugate targeting immune checkpoint. |
All AbMole products are for research use only, cannot be used for human consumption or veterinary use. We do not provide products or services to individuals. Please comply with the intended use and do not use AbMole products for any other purpose.


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2024 AbMole BioScience. All Rights Reserved.
