All AbMole products are for research use only, cannot be used for human consumption.
For this product's availability, delivery time and price, please email [email protected] directly or click the "Inquiry Now" button below.
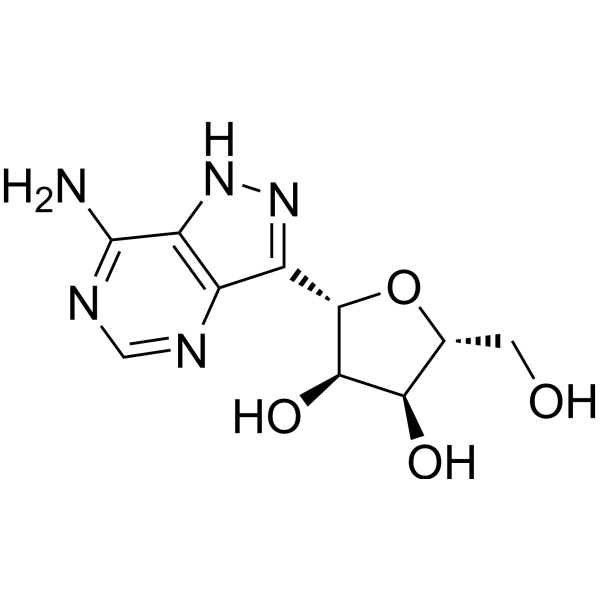
Formycin A (NSC 102811), a purine nucleoside antibiotic, is a potent human immunodeficiency virus type 1 (HIV-1) inhibitor with an EC50 of 10 μM. Formycin A shows antitumor and antiviral activities.
| Molecular Weight | 267.24 |
| Formula | C10H13N5O4 |
| CAS Number | 6742-12-7 |
| Form | Solid |
| Storage |
Powder -20°C 3 years ; 4°C 2 years In solvent -80°C 6 months ; -20°C 1 month |
| Related HIV Protease Products |
|---|
| Ritonavir-13c,d3
Ritonavir-13c,d3 |
| Arg-Val-(Nle-p-nitro)-Phe-Glu-Ala-Nle-NH2
Arg-Val-(Nle-p-nitro)-Phe-Glu-Ala-Nle-NH2 is a fluorogenic substrate of HIV-1 protease. |
| Ac-Ser-Gln-Asn-Tyr-Pro-Val-Val-NH2
Ac-Ser-Gln-Asn-Tyr-Pro-Val-Val-NH2 is a substrato peptídico of HIV-1 protease. |
| HIV-1, HIV-2 Protease Substrate
HIV-1, HIV-2 Protease Substrate is the substrate of HIV-1, HIV-2 protease. |
| HIV Protease Substrate IV
HIV Protease Substrate IV is a substrate of HIV protease. |
All AbMole products are for research use only, cannot be used for human consumption or veterinary use. We do not provide products or services to individuals. Please comply with the intended use and do not use AbMole products for any other purpose.


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2024 AbMole BioScience. All Rights Reserved.
