All AbMole products are for research use only, cannot be used for human consumption.
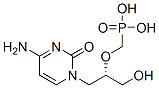
Cidofovir (Vistide) is an injectable antiviral medication for the treatment of cytomegalovirus (CMV) retinitis (IC50 = 0.94 μM). Cidofovir suppresses CMV replication by selective inhibition of viral DNA polymerase and therefore prevention of viral replication and transcription.

Antiviral Res. 2020 Apr;176:104754.
Acyclovir, Cidofovir, and Amenamevir Have Additive Antiviral Effects on Herpes Simplex Virus TYPE 1
Cidofovir purchased from AbMole
| Cell Experiment | |
|---|---|
| Cell lines | Madin–Darby Canine Kidney or Vero cells |
| Preparation method | In vitro efficacy of cidofovir against CHV-1 Madin–Darby Canine Kidney or Vero cells were plated into 96-well plates at 1*104 cells/well and maintained in Dulbecco’s minimal essential medium with 1 g/L glucose, l-glutamine, and sodium pyruvate (Cell Grow; Corning, Manassas, VA) containing 10% fetal bovine serum (Atlanta Biological, Flowery Branch, GA) and penicillin (200 U/mL)/ streptomycin (200 mg/mL) (Life Technologies, Grand Island, NY) for 24 h until confluent. Monolayers were infected with 5 PFU/well CHV-1 (a previously described field strain that was also utilized during the in vivo experimental study evaluating cidofovir) or HSV-1 strain F, respectively. After 2 h of incubation at 37 C, virus supernatants were removed, cells were rinsed once with phosphate-buffered saline (PBS), and 100 mL of sequential 2-fold serial dilutions of cidofovir (starting at 625 mM) was added to each well. Infection controls without cidofovir and noninfected drug-treated controls were also included. Plates were incubated at 37 C until a cytopathic effect (CPE) was visible in the infected nontreated controls, which was *72 hours postinfection (hpi) for CHV-1 and 48 hpi for HSV-1, respectively. Plates were rinsed 3 times with PBS, fixed in 80% ethanol for 10 min at -20 C, and stained with crystal violet (Fisher Scientific, Fair Lawn, NJ). Virusinduced CPE was scored as the percent of wells per dilution with observable plaque formation, and EC50 values were calculated using GraphPad Prism. |
| Concentrations | 0~625µM |
| Incubation time | 72 hours postinfection (hpi) for CHV-1 and 48 hpi for HSV-1 |
| Animal Experiment | |
|---|---|
| Animal models | Recurrent ocular CHV-1 infection in dogs by administration of systemic prednisolone (3.0 mg/kg PO q24 h) for 7 consecutive days beginning on study day 1. |
| Formulation | 0.9% sodium chloride solution |
| Dosages | 1 drop of 0.5% cidofovir ophthalmic solution |
| Administration | eyes drop |
| Molecular Weight | 279.19 |
| Formula | C8H14N3O6P |
| CAS Number | 113852-37-2 |
| Solubility (25°C) | DMSO |
| Storage |
Powder -20°C 3 years ; 4°C 2 years In solvent -80°C 6 months ; -20°C 1 month |
| Related Anti-infection Products |
|---|
| Strictinin
Strictinin is a phenolic compound which has potential antiviral, antibacterial and laxative activities. Strictinin occurs by accelerating intestinal transit rather than enhancing gastric emptying, increasing food intake, or inducing diarrhea in rats. |
| Torcitabine
Torcitabine (2'-Deoxy-L-cytidine) is an antiviral agent. Torcitabine has the potential for chronic hepatitis B virus infection research. Torcitabine (2'-Deoxy-L-cytidine) shows greater inhibition of first strand than second strand DNA synthesis. |
| ABMA
ABMA is a broad-spectrum inhibitor of intracellular toxins and pathogens. ABMA efficiently protects cells against various toxins and pathogens including viruses, intracellular bacteria and parasite. |
| DDX3-IN-1
DDX3-IN-1 is a DEAD-box polypeptide 3 (DDX3) inhibitor with CC50 values of 50 and 36 μM for HIV and HCV, respectively. DDX3-IN-1 has antiviral activity. |
| K22
K22 is an inhibitor of coronavirus RNA synthesis that specifically targets membrane-associated coronavirus RNA synthesis. K22 effectively blocks replication of multiple coronaviruses by inhibiting the critical step of viral replication complex anchoring to host cell membranes to form double-membrane vesicles (DMVs). K22 exhibits broad-spectrum antiviral activity against diverse coronaviruses. |
All AbMole products are for research use only, cannot be used for human consumption or veterinary use. We do not provide products or services to individuals. Please comply with the intended use and do not use AbMole products for any other purpose.


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2024 AbMole BioScience. All Rights Reserved.
