All AbMole products are for research use only, cannot be used for human consumption.
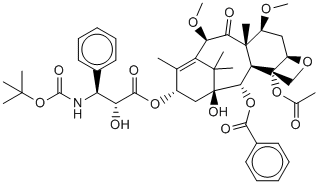
Cabazitaxel (Jevtana) is a semi-synthetic derivative of a natural taxoid. Cabazitaxel (Jevtana) increases CYP3A enzyme activities in rat hepatocytes. The mean ex-vivo human plasma protein binding of Cabazitaxel (Jevtana) is 91.6%. Cabazitaxel (Jevtana) is rapidly and extensively metabolised into numerous metabolites. Cabazitaxel (Jevtana) demonstrates activity in several murine and human resistant cell lines. With a 4-day exposure to cabazitaxel, cytotoxicity is noted with relatively low cabazitaxel concentrations. Cabazitaxel (Jevtana) shows high antitumor activity in 3 human colorectal cell lines (HCT-116, HCT-8, and HT-29). In accompanying models, Cabazitaxel (Jevtana) has significant antitumor activity. In murine tumor xenografts (colon C38 and pancreas P03), Cabazitaxel (Jevtana) causes complete tumor regressions. Using SF-295 and U251 human glioblastoma cell lines, both orthotopic and subcutaneous murine xenografts are generated. Cabazitaxel (Jevtana) treatment leads to complete regression in the majority of subcutaneously implanted tumors. Cabazitaxel (Jevtana) has entered in a phase I clinical trial in the treatment of solid cancer. Cabazitaxel (Jevtana) plus Prednisone, Carboplatin has entered in a phase II clinical trial in the treatment of prostate cancer. Cabazitaxel is a semi-synthetic derivative of a natural taxoid.
| Cell Experiment | |
|---|---|
| Cell lines | MCF-7 cells |
| Preparation method | Parallel drug selections were initiated using 0.1 nmol/L cabazitaxel, a concentration that would inhibit growth in MCF-7 cells by 50% (IC50 value) with and without 2 μmol/L PSC-833. Selections continued by increasing the drug concentration in a stepwise manner up to a final concentration of 5 nmol/L cabazitaxel. |
| Concentrations | 5 nmol/L |
| Incubation time | 72 h |
| Animal Experiment | |
|---|---|
| Animal models | Male nude mice |
| Formulation | 1% medium /0.1% Tween-20 |
| Dosages | 30 mg/kg |
| Administration | i.v. |
| Molecular Weight | 835.93 |
| Formula | C45H57NO14 |
| CAS Number | 183133-96-2 |
| Solubility (25°C) | DMSO 90 mg/mL |
| Storage |
Powder -20°C 3 years ; 4°C 2 years In solvent -80°C 6 months ; -20°C 1 month |
[2] Duran GE, et al. Mol Cancer Ther. Mechanisms of resistance to cabazitaxel.
| Related Products |
|---|
| Myoglobin (from equine skeletal muscle)
Myoglobin is a small molecular pigment protein formed by binding globin to Heme, which can be reversibly bound to oxygen to form MbO2, MbO2 is called oxymyoglobin, and Mb is called deoxymyoglobin. Myoglobin has the role of transporting and storing oxygen in muscle cells. |
| Hemoglobin (from bovine blood)
Hemoglobin is a iron-containing protein in red blood cells with oxygen binding properties. Hemoglobin is an inducer of HO-1. Hemoglobin consits of heme, which binds to oxygen. Hemoglobin also transports other gases, such as carbon dioxide, nitric oxide, hydrogen sulfide and sulfide. |
| Diethylenetriaminepentaacetic dianhydride
Diethylenetriaminepentaacetic dianhydride (DTPA anhydride) is a bifunctional chelator whose anhydride can react with amino groups in proteins (such as lysine residues) to form stable amide bonds. Diethylenetriaminepentaacetic dianhydride (DTPA anhydride) can also bind to radionuclides to synthesize radionuclide-labeled drug conjugates (RDCs). |
| 3-Phenylthiophene
3-Phenylthiophene is a biochemical material that can be used in scientific research. 3-Phenylthiophene is a conducting polymer precursor. |
| DSPE-PEG-FA
DSPE-PEG2K-FA is a PEG derivative containing folic acid. DSPE-PEG2K-FA has a targeting effect and can bind to folic acid receptors in cancer cells. DSPE-PEG2K-FA forms micelles/lipid bilayers and can be used in research on targeted drug delivery systems. |
All AbMole products are for research use only, cannot be used for human consumption or veterinary use. We do not provide products or services to individuals. Please comply with the intended use and do not use AbMole products for any other purpose.


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2024 AbMole BioScience. All Rights Reserved.
