All AbMole products are for research use only, cannot be used for human consumption.
For this product's availability, delivery time and price, please email [email protected] directly or click the "Inquiry Now" button below.
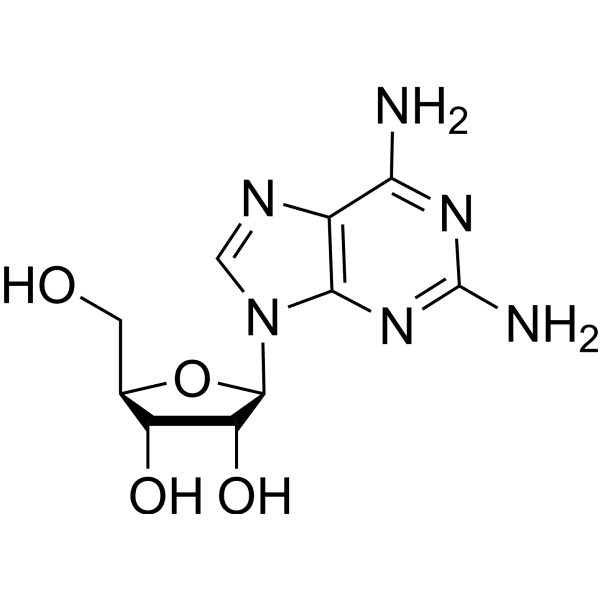
2-Aminoadenosine is an adenosine analog.
| Molecular Weight | 282.26 |
| Formula | C10H14N6O4 |
| CAS Number | 2096-10-8 |
| Form | Solid |
| Storage | 4°C, protect from light |
[4] K Grzeskowiak et al. J Mol Evol. Template-directed synthesis with 2-aminoadenosine
| Related Nucleoside Antimetabolite/Analog Products |
|---|
| 5-Bromouridine
5-Bromouridine is a purine nucleoside analog, with broad antitumor activity rely on inhibition of DNA synthesis, induction of apoptosis, etc. |
| 6-Azathymine
6-Azathymine, a 6-nitrogen analog of thymine, is a potent D-3-aminoisobutyrate-pyruvate aminotransferase inhibitor. |
| N1-Methylpseudouridine
N1-methyl-pseudouridine (1-Methylpseudouridine), a methylpseudouridine, outperforms 5 mC and 5 mC/N1-methyl-pseudouridine in translation. |
| Diguanosine 5′-triphosphate
Diguanosine 5′-triphosphate (Gp3G) is a dinucleoside triphosphates. |
| Gemcitabine elaidate hydrochloride
Gemcitabine elaidate (CP-4126) hydrochloride is lipophilic pro-agent of Gemcitabine. |
All AbMole products are for research use only, cannot be used for human consumption or veterinary use. We do not provide products or services to individuals. Please comply with the intended use and do not use AbMole products for any other purpose.


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2024 AbMole BioScience. All Rights Reserved.
