All AbMole products are for research use only, cannot be used for human consumption.
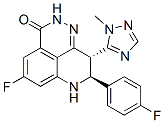
Talazoparib (BMN-673) is a highly potent, orally active PARP1/2 inhibitor.Talazoparib inhibits PARP1 and PARP2 enzyme activity with Kis of 1.2 nM and 0.87 nM, respectively. Talazoparib shows EC50s of 0.3 nM, 5 nM and 0.31 for MX-1 cells (BRCA1 mutant), Capan-1 cells (BRCA2 mutant) and MRC-5 cells (normal).
In vivo, Talazoparib (0.33 mg/kg; i.g.; once daily; for 28 days) exhibits antitumor activity against BRCA1 mutant breast cancer model in mice

Cancers. 2023 Aug 16.
Development of a Patient-Derived 3D Immuno-Oncology Platform to Potentiate Immunotherapy Responses in Ascites-Derived Circulating Tumor Cells
Talazoparib purchased from AbMole

Front Oncol. 2023 May 9;13:1175617.
Sustained delivery of PARP inhibitor Talazoparib for the treatment of BRCA-deficient ovarian cancer
Talazoparib purchased from AbMole

Front Oncol. 2022 Aug 16;12:940377.
Effectiveness of PARP inhibition in enhancing the radiosensitivity of 3D spheroids of head and neck squamous cell carcinoma
Talazoparib purchased from AbMole

BMC Biol. 2021 May 20;19(1):108.
Very long intergenic non-coding (vlinc) RNAs directly regulate multiple genes in cis and trans
Talazoparib purchased from AbMole

Clin Cancer Res. 2020 Oct 15;26(20):5462-5476.
A Preclinical Trial and Molecularly Annotated Patient Cohort Identify Predictive Biomarkers in Homologous Recombination-deficient Pancreatic Cancer
Talazoparib purchased from AbMole

Nat Commun. 2019 Dec 20;10(1):5799.
Novel approach reveals genomic landscapes of single-strand DNA breaks with nucleotide resolution in human cells.
Talazoparib purchased from AbMole

Proc Natl Acad Sci U S A. 2019 Nov 5;116(45):22609-22618.
DNA methyltransferase inhibitors induce a BRCAness phenotype that sensitizes NSCLC to PARP inhibitor and ionizing radiation.
Talazoparib purchased from AbMole


Patent. US10363264B2 2019-07-30.
Patent. US10363264B2
Talazoparib purchased from AbMole

Patent. US2016346306A1 2019-07-30.
Patent. US2016346306A1
Talazoparib purchased from AbMole

Nat Commun. 2018 Nov 12;9(1):4760.
Defective DNA damage repair leads to frequent catastrophic genomic events in murine and human tumors.
Talazoparib purchased from AbMole

University of Maryland. 2017.
Developing a Novel Combination Therapy and Elucidating Mechanisms of Increased ALT-NHEJ in Acute Myeloid Leukemia
Talazoparib purchased from AbMole

Cancer Cell. 2016 Oct 10;30(4):637-650.
Enhancing the Cytotoxic Effects of PARP Inhibitors with DNA Demethylating Agents – A Potential Therapy for Cancer
Talazoparib purchased from AbMole

Patent. US2016367581A1 2016 Dec 22.
Patent. US2016367581A1
Talazoparib purchased from AbMole
 polymerase-12 inhibitor BMN 673-1 August 2015.png)
Cancer Lett. 2015 Aug 1;364(1):8-16.
Increased in vitro and in vivo sensitivity of BRCA2-associated pancreatic cancer to the poly(ADP-ribose) polymerase-1/2 inhibitor BMN 673.
Talazoparib purchased from AbMole

Cell Rep. 2014 Nov 6;9(3):829-41.
Targeting the DNA repair pathway in Ewing sarcoma.
Talazoparib purchased from AbMole
| Cell Experiment | |
|---|---|
| Cell lines | Capan-1 and MIA PaCa-2 |
| Preparation method | Real-time cell analysis (xCELLigence) |
| Concentrations | |
| Incubation time | 48 h |
| Animal Experiment | |
|---|---|
| Animal models | Patient-derived xenograft (PDX) model |
| Formulation | Solubilized in DMSO and diluted with PBS containing 10% dimethylacetamide (Sigma-Aldrich) and 6% Solutol (Sigma-Aldrich). |
| Dosages | 0.33 mg/kg, 0.05 cc, once daily |
| Administration | oral gavage |
| Molecular Weight | 380.35 |
| Formula | C19H14F2N6O |
| CAS Number | 1207456-01-6 |
| Solubility (25°C) | DMSO 25 mg/mL |
| Storage |
Powder -20°C 3 years ; 4°C 2 years In solvent -80°C 6 months ; -20°C 1 month |
| Related PARP Products |
|---|
| 2-Methylquinazolin-4-ol
2-Methylquinazolin-4-ol is a potent competitive poly(ADP-ribose) synthetase inhibitor, with a Ki of 1.1 μM. 2-Methylquinazolin-4-ol mammalian aspartate transcarbamylase (ATCase) inhibitor, with IC50 of 0.20 mM. |
| DPQ
DPQ is a potent PARP-1 inhibitor. |
| OUL232
OUL232 is a potent inhibitor of mono-ARTs PARP7, PARP10, PARP11, PARP12, PARP14, and PARP15. |
| AZ3391
AZ3391 is a potent inhibitor of PARP. |
| PARP1-IN-5
PARP1-IN-5 is a low toxicity, orally active, potent and selective PARP-1 inhibitor (IC50 =14.7 nM). |
All AbMole products are for research use only, cannot be used for human consumption or veterinary use. We do not provide products or services to individuals. Please comply with the intended use and do not use AbMole products for any other purpose.


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2024 AbMole BioScience. All Rights Reserved.
