All AbMole products are for research use only, cannot be used for human consumption.
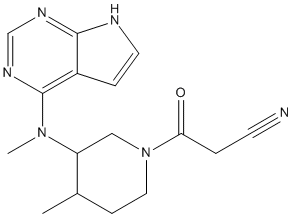
Tofacitinib (CP-690550) is a potent, small molecule inhibitor of JAK-3, which exhibiting potent effects in preclinical transplantation and arthritis models. It displays greater antiproliferative and pro-apoptotic activity against murine multipotent factor-dependent cell Patersen-erythropoietin receptor (FDCP-EpoR) cells harboring JAK2(V617F) compared with JAK2(WT). JAK-3 has been shown to play a key role in cytokine signaling via gammac, e.g. IL-2, 4, 7, 9, 15, 21. In vitro, Tofacitinib effectively inhibited a murine mixed lymphocyte reaction (MLR) (IC50= 91 nm). Mice chronically dosed with CP-690550 (1.5-15 mg/kg/day) demonstrated dose- and time-dependent alterations in lymphocyte subsets when examined by flow cytometry. The most dramatic change observed was a 96% reduction in splenic NK1.1 + TCRbeta- cell numbers following 21 days of treatment. Delayed-type hypersensitivity (DTH) responses in sensitized mice were reduced in a dose-dependent manner following treatment with the JAK-3 inhibitor (1.87-30 mg/kg, s.c.). Extended survival of neonatal Balb/c hearts implanted into the ear pinna of MHC mismatched C3H/HEN mice was observed with CP-690550 monotherapy (10-30 mg/kg/day), but improved upon combination with cyclosporin (10 mg/kg/day). Furthermore, the ability of Tofacitinib to extend cardiac allograft survival in murine models suggests it may afford a new treatment for prevention of transplant rejection.

Clin Rheumatol. 2025 May 29; .
Exploring the potential mechanism of tofacitinib therapy for ankylosing spondylitis through gut microbiome and plasma metabolomics
Tofacitinib purchased from AbMole

J Cell Mol Med. 2024 Apr 20;28(7):e18190.
Microglia orchestrate synaptic and neuronal stripping: Implication in neuropsychiatric lupus
Tofacitinib purchased from AbMole

Patent. US12138490B2 2024 Nov 12.
Patent. US12138490B2
Tofacitinib purchased from AbMole

Patent. US2023043374A1 2023 Feb 09.
Patent. US2023043374A1
Tofacitinib purchased from AbMole

EMBO Mol Med. 2022 Aug 8;14(8):e15653.
Single‐cell transcriptomics reveals a senescence‐associated IL‐6/CCR6 axis driving radiodermatitis
Tofacitinib purchased from AbMole

Front Immunol. 2022 May 23;13:866638.
A Novel STAT3 Gain-of-Function Mutation in Fatal Infancy-Onset Interstitial Lung Disease
Tofacitinib purchased from AbMole

Front Immunol. 2021 Jul 29;12:675542.
Tofacitinib Ameliorates Lupus Through Suppression of T Cell Activation Mediated by TGF-Beta Type I Receptor
Tofacitinib purchased from AbMole

J Virol. 2020 Feb 14;94(5):e01791-19.
Targeting Kaposi's Sarcoma-Associated Herpesvirus ORF21 Tyrosine Kinase and Viral Lytic Reactivation by Tyrosine Kinase Inhibitors Approved for Clinical Use
Tofacitinib purchased from AbMole

Cell Stem Cell. 2019 Apr 4;24(4):654-669.e6.
A Subset of TREM2+ Dermal Macrophages Secretes Oncostatin M to Maintain Hair Follicle Stem Cell Quiescence and Inhibit Hair Growth.
Tofacitinib purchased from AbMole

Patent. US10265258B2 2019 Apr 23.
Patent. US10265258B2
Tofacitinib purchased from AbMole

Arch Dermatol Res. 2018 Oct 5.
Effect of tofacitinib on the expression of noggin/BMP-4 and hair growth stimulation in mice
Tofacitinib purchased from AbMole

Patent. JP2018027959A 2018 Feb 22.
Patent. JP2018027959A
Tofacitinib purchased from AbMole

Patent. US2018291378A1 2018 Oct 11.
Patent. US2018291378A1
Tofacitinib purchased from AbMole

Arch Dermatol Res. 2017 Sep 7.
Efficacy of topical tofacitinib in promoting hair growt in non‑scarring alopecia: possible mechanism via VEGF induction
Tofacitinib purchased from AbMole

TJPS. 2017;41 (Supplement Issue): 225-228.
Topical tofacitinib reduce expression of BMP-4 in mouse hair follicle
Tofacitinib purchased from AbMole

Patent. JP6212107B2 2017 Oct 11.
Patent. JP6212107B2
Tofacitinib purchased from AbMole

Patent. WO2016179605A1 2016 Nov 10.
Patent. WO2016179605A1
Tofacitinib purchased from AbMole

Sci Adv. 2015 Oct 23.
Pharmacologic inhibition of JAK-STAT signaling promotes hair growth.
Tofacitinib purchased from AbMole
Nat Med. 2014 Sep;20(9):1043-9.
Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition.
Tofacitinib purchased from AbMole
| Cell Experiment | |
|---|---|
| Cell lines | cytokine-dependent NK92 cell line |
| Preparation method | (A) After cytokine starvation for 24 hours, NK92 cells were stimulated by the addition of human IL-2 for 48 hours with and without the addition of serially increasing concentrations of tofacitinib. 3H-thymidine was added during the last 6 hours of the cultures. Cells were then harvested and analyzed for 3H-thymidine incorporation. (B) Cytokine-starved NK92 cells were stimulated with human IL-2, combined IL-6/IL- 6R, or IL-12 for 48 hours with and without serially increasing concentrations of tofacitinib. (C) The NK92 cells were treated as those in panel B with and without a single 50nM dose of tofacitinib. Data are presented as means ±SD (A-C) and are representative of 3 independent experiments. (D) After cytokine starvation for 24 hours, NK92 cells were stimulated with 30 ng/mL of IL-2, 100 ng/mL of combined IL-6/IL-6R, or 100 ng/mL of IL-12 for 1 hour with and without the addition of tofacitinib. The cell lysates were immunoblotted with an anti–phospho-STAT5 monoclonal antibody and an anti-STAT5 antibody. ß-Actin was used as an input control. Data are representative of 3 independent experiments. |
| Concentrations | 0~400 nM |
| Incubation time | 48 h |
| Animal Experiment | |
|---|---|
| Animal models | IL-15–transgenic CD8 T-cell leukemia–bearing mice |
| Formulation | dissolved in polyethylene glycol 300 (PEG300; VWR Scientific Products) |
| Dosages | 50 mg/mL |
| Administration | continuously administered via a subcutaneous mini-osmotic pump (ALZET) |
| Molecular Weight | 312.37 |
| Formula | C16H20N6O |
| CAS Number | 477600-75-2 |
| Solubility (25°C) | DMSO ≥60 mg/mL |
| Storage | -20°C, sealed |
| Related JAK Products |
|---|
| iJak-381
iJak-381 is a JAK1/2 inhibitor with anti-inflammatory activity. |
| Ritlecitinib (malonate)
Ritlecitinib (PF-06651600) malonate is an orally active and selective JAK3 inhibitor with an IC50 of 33.1 nM. |
| Deuruxolitinib
Deuruxolitinib is a deuterated Ruxolitinib (INCB18424), which modulates the activity of JAK1/JAK2. Deuruxolitinib can be used for the research of hair loss disorders. |
| A-005
A-005 is a potentially first-in-class, brain-penetrating TYK2 allosteric inhibitor for studies related to neuroinflammatory and neurodegenerative diseases. |
| Tyrosine Protein Kinase JAK 2 (Phospho-Tyr8, 9)
Tyrosine Protein Kinase JAK 2 (Phospho-Tyr8, 9) is a peptide corresponding to amino acids 475 to 491 of mouse JAK2. |
All AbMole products are for research use only, cannot be used for human consumption or veterinary use. We do not provide products or services to individuals. Please comply with the intended use and do not use AbMole products for any other purpose.


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2024 AbMole BioScience. All Rights Reserved.
