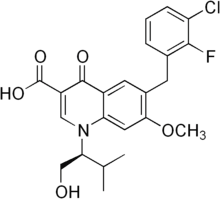
Elvitegravir also inhibits PBMC and PA with IC50 of 0.89 and 20 nM, respectively. Elvitegravir (also known as JTK-303/GS-9137) blocked the integration of HIV-1 cDNA through the inhibition of DNA strand transfer. EVG inhibited the replication of HIV-1, including various subtypes and multiple-drug-resistant clinical isolates, and HIV-2 strains with a 50% effective concentration in the subnanomolar to nanomolar range. The replication capacity of EVG-resistant variants was significantly reduced relative to both wild-type virus and other IN inhibitor-resistant variants selected by L-870810. Elvitegravir (GS-9137) is active against HIV-1 and HIV-2 and has a serum-free antiviral IC50 of 0.3-0.9 nM in peripheral blood mononuclear cells. According to the results of the phase II clinical trial, patients taking once-daily elvitegravir boosted by ritonavir had greater reductions in viral load after 24 weeks compared to individuals randomized to receive a ritonavir-boosted protease inhibitor. Elvitegravir is currengly in a Phase III clinical trial in the treatment of HIV-1 Infection.
| Molecular Weight | 447.88 |
| Formula | C23H23ClFNO5 |
| CAS Number | 697761-98-1 |
| Solubility (25°C) | DMSO 90 mg/mL Ethanol 30 mg/mL |
| Storage |
Powder -20°C 3 years ; 4°C 2 years In solvent -80°C 6 months ; -20°C 1 month |
| Species | Mouse | Rat | Rabbit | Guinea pig | Hamster | Dog |
| Weight (kg) | 0.02 | 0.15 | 1.8 | 0.4 | 0.08 | 10 |
| Body Surface Area (m2) | 0.007 | 0.025 | 0.15 | 0.05 | 0.02 | 0.5 |
| Km factor | 3 | 6 | 12 | 8 | 5 | 20 |
| Animal A (mg/kg) = Animal B (mg/kg) multiplied by | Animal B Km |
| Animal A Km |
For example, to modify the dose of Compound A used for a mouse (20 mg/kg) to a dose based on the BSA for a rat, multiply 20 mg/kg by the Km factor for a mouse and then divide by the Km factor for a rat. This calculation results in a rat equivalent dose for Compound A of 10 mg/kg.
| Related Integrase Products |
|---|
| Bictegravir Sodium
Bictegravir (GS-9883) Sodium is a novel and potent inhibitor of HIV-1 integrase, specifically targets IN strand transfer activity with an IC50 of 7.5 nM. |
| Lavendustin B
Lavendustin B inhibits HIV-1 integrase (IN) interaction with its cognate cellular cofactor lens epithelium-derived growth factor (LEDGF/p75). Lavendustin B is an inhibitor of Tyrosine Kinase and also a competitive inhibitor of glucose transporter 1 (Glut1). |
| Bictegravir
Bictegravir (GS-9883) is a novel, potent inhibitor of HIV-1 integrase with an IC50 of 7.5 nM. |
| Raltegravir potassium
Raltegravir potassium (MK0518 potassium) is a potent integrase (IN) inhibitor, used to treat HIV infection. |
| Dolutegravir sodium
Dolutegravir sodium is a potent, second generation HIV integrase inhibitor (IC₅₀ = 2.7 nM) that displays potent antiviral activity. |


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2023 AbMole BioScience. All Rights Reserved.
