All AbMole products are for research use only, cannot be used for human consumption.
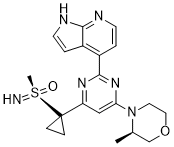
Ceralasertib (AZD6738) is an orally active, selective ATR kinase inhibitor with an IC50 of 1 nM. AZD6738 inhibits ATR kinase activity and impels cell viability. AZD6738 induces rapid cell death in tumor cells with an ATM gene defect.
In vitro studies showed that AZD6738 inhibited ATR kinase activity and impaired cell activity in four Kras mutant cell lines H23, H460, A549 and H358. In ATM deficient H23 cells, AZD6738 strongly enhanced the effect of cisplatin on rapid cell death. In cells with p53 or ATM deletion, AZD6738 treatment causes replication fork stagnation and accumulation of unrepaired DNA damage, leading to mitosis disorder and thus cell death.
In vivo studies, AZD6738 (50 mg/kg, P.O.) induced tumor growth inhibition (TGI) in nude mice burdened with H460 and H23 tumors, and combined with cisplatin induced rapid degeneration of ATM-deficient H23 tumors. The combination of AZD6738 (50 mg/kg)+IR (2 Gy) in nude mice loaded with LoVo xenografts avoided toxicity while maintaining efficacy.
| Cell Experiment | |
|---|---|
| Cell lines | H23, H460, A549, and H358 cells |
| Preparation method | Cells are treated in white walled, clear bottom 96-well plates with the indicated doses of AZD6738, cisplatin, gemcitabine, or combination for 48 h. ATP levels are assessed as surrogate measure of viability is assessed using the CellTiter-Glo Luminescent Cell Viability Assay and Safire2 plate reader. Raw data are corrected for background luminescence prior to further analysis. For AZD6738 treatment, log dose response curves are generated in GraphPad Prism 6 by nonlinear regression (log(inhibitor) vs. response with variable slope) of log-transformed (x = log(x)) data normalized to the mean of untreated controls. GI50 values, defined as the dose X at which Y = 50%, were extrapolated from dose response curves. |
| Concentrations | ~30 μM |
| Incubation time | 48 h |
| Animal Experiment | |
|---|---|
| Animal models | Female athymic nude mice bearing H23 or H460 xenografts |
| Formulation | 10% DMSO, 40% propylene glycol, and 50% sterile dH2O |
| Dosages | 25 or 50 mg/kg |
| Administration | p.o. |
| Molecular Weight | 412.51 |
| Formula | C20H24N6O2S |
| CAS Number | 1352226-88-0 |
| Solubility (25°C) | DMSO: 70 mg/mL Ethanol: 40 mg/mL warmed |
| Storage |
Powder -20°C 3 years ; 4°C 2 years In solvent -80°C 6 months ; -20°C 1 month |
| Related ATM/ATR Products |
|---|
| ART0380
ART0380 is a potent and selective ATR kinase inhibitor. |
| KU 59403
KU 59403 is a potent ATM inhibitor, with IC50 values of 3 nM, 9.1 μM and 10 μM for ATM, DNA-PK and PI3K, respectively. |
| SKLB-197
SKLB-197 showed an IC50 value of 0.013 μM against ATR but very weak or no activity against other 402 protein kinases. |
| ATR-IN-4
ATR-IN-4 is a potent ATR (Ataxia telangiectasia mutated gene Rad 3-associated kinase) inhibitor. |
| M3541
M3541 is a potent, ATP-competitive and selective ATM inhibitor with an IC50 of 0.25 nM. |
All AbMole products are for research use only, cannot be used for human consumption or veterinary use. We do not provide products or services to individuals. Please comply with the intended use and do not use AbMole products for any other purpose.


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2024 AbMole BioScience. All Rights Reserved.
