Cilastatin attenuates VAN-induced acute renal failure in vitro by decreasing apoptosis without affecting antibacterial activity. This effect could be related, at least in part, to the reduction in accumulation of the drug in cells. Therefore, cilastatin could represent a novel therapeutic approach in reducing VAN-induced renal damage without compromising bactericidal efficacy.
| Cell Experiment | |
|---|---|
| Cell lines | Proximal Tubular Primary Cell |
| Preparation method | Cells were pretreated with VAN (0.6, 3, and 6 mg/mL) alone or in combination with cilastatin (200 μg/mL) before being trypsinized, washed twice with PBS, and incubated for 30 min in the dark in 100 μL buffer containing 5 μL fluorescein isothiocyanate- (FITC-) labeled annexin V and 5 μL PI for flow cytometry. |
| Concentrations | 200 μg/mL |
| Incubation time | 24 h |
| Animal Experiment | |
|---|---|
| Animal models | |
| Formulation | |
| Dosages | |
| Administration | |
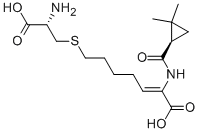
| Molecular Weight | 358.45 |
| Formula | C16H26N2O5S |
| CAS Number | 82009-34-5 |
| Solubility (25°C) | DMSO ≥ 35 mg/mL |
| Storage |
Powder -20°C 3 years ; 4°C 2 years In solvent -80°C 6 months ; -20°C 1 month |
| Species | Mouse | Rat | Rabbit | Guinea pig | Hamster | Dog |
| Weight (kg) | 0.02 | 0.15 | 1.8 | 0.4 | 0.08 | 10 |
| Body Surface Area (m2) | 0.007 | 0.025 | 0.15 | 0.05 | 0.02 | 0.5 |
| Km factor | 3 | 6 | 12 | 8 | 5 | 20 |
| Animal A (mg/kg) = Animal B (mg/kg) multiplied by | Animal B Km |
| Animal A Km |
For example, to modify the dose of Compound A used for a mouse (20 mg/kg) to a dose based on the BSA for a rat, multiply 20 mg/kg by the Km factor for a mouse and then divide by the Km factor for a rat. This calculation results in a rat equivalent dose for Compound A of 10 mg/kg.
| Related Products |
|---|
| PRGL493
PRGL493 is a potent and selective long-chain acyl-CoA synthetase 4 (ACSL4) inhibitor. PRGL493 blocks cell proliferation and tumor growth in both breast and prostate cellular and animal models. |
| Hercynine
Hercynine is a natural product, found in the fine leaf algae, Schizosaccharomyces cerevisiae and honey bees, and is a precursor of L-(+)-ergothioneine. |
| C24-Ceramide
C24 Ceramide (Lignoceric ceramide) is a naturally occurring ceramide and an integral part of the sphingomyelin cycle. C24 Ceramide (Lignoceric ceramide) stimulates the proliferation of basophilic erythroblasts, suggesting a possible role in erythrocyte maturation. |
| RNA-protein crosslinking (sulfhydryl addition) agent
RNA-protein crosslinking (sulfhydryl addition) agent is a thiolating agent that is useful in cross-linking proteins. Thiolan-2-imine hydrochloride is a reagent for the introduction of sulphydryl groups into oligosaccharides derived from asparagine-linked glycans. |
| Izeltabart
Izeltabart is a high-affinity humanized antibody targeting ADAM9. Izeltabart can be used as ADC Antibody for site-specific conjugation with Maytansinoid-based DM21-C to synthesize IMGC936, an Antibody-drug Conjugate with strong anti-cancer activity. IMGC936 exhibits cytotoxicity against ADAM9-positive human tumor cell lines and potent antitumor activity in xenograft tumor models. |


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2023 AbMole BioScience. All Rights Reserved.
