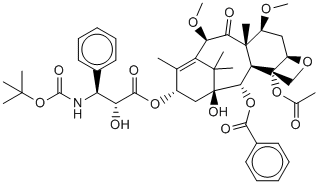
Cabazitaxel (Jevtana) is a semi-synthetic derivative of a natural taxoid. Cabazitaxel (Jevtana) increases CYP3A enzyme activities in rat hepatocytes. The mean ex-vivo human plasma protein binding of Cabazitaxel (Jevtana) is 91.6%. Cabazitaxel (Jevtana) is rapidly and extensively metabolised into numerous metabolites. Cabazitaxel (Jevtana) demonstrates activity in several murine and human resistant cell lines. With a 4-day exposure to cabazitaxel, cytotoxicity is noted with relatively low cabazitaxel concentrations. Cabazitaxel (Jevtana) shows high antitumor activity in 3 human colorectal cell lines (HCT-116, HCT-8, and HT-29). In accompanying models, Cabazitaxel (Jevtana) has significant antitumor activity. In murine tumor xenografts (colon C38 and pancreas P03), Cabazitaxel (Jevtana) causes complete tumor regressions. Using SF-295 and U251 human glioblastoma cell lines, both orthotopic and subcutaneous murine xenografts are generated. Cabazitaxel (Jevtana) treatment leads to complete regression in the majority of subcutaneously implanted tumors. Cabazitaxel (Jevtana) has entered in a phase I clinical trial in the treatment of solid cancer. Cabazitaxel (Jevtana) plus Prednisone, Carboplatin has entered in a phase II clinical trial in the treatment of prostate cancer. Cabazitaxel is a semi-synthetic derivative of a natural taxoid.
| Cell Experiment | |
|---|---|
| Cell lines | MCF-7 cells |
| Preparation method | Parallel drug selections were initiated using 0.1 nmol/L cabazitaxel, a concentration that would inhibit growth in MCF-7 cells by 50% (IC50 value) with and without 2 μmol/L PSC-833. Selections continued by increasing the drug concentration in a stepwise manner up to a final concentration of 5 nmol/L cabazitaxel. |
| Concentrations | 5 nmol/L |
| Incubation time | 72 h |
| Animal Experiment | |
|---|---|
| Animal models | Male nude mice |
| Formulation | 1% medium /0.1% Tween-20 |
| Dosages | 30 mg/kg |
| Administration | i.v. |
| Molecular Weight | 835.93 |
| Formula | C45H57NO14 |
| CAS Number | 183133-96-2 |
| Solubility (25°C) | DMSO 90 mg/mL |
| Storage |
Powder -20°C 3 years ; 4°C 2 years In solvent -80°C 6 months ; -20°C 1 month |
| Species | Mouse | Rat | Rabbit | Guinea pig | Hamster | Dog |
| Weight (kg) | 0.02 | 0.15 | 1.8 | 0.4 | 0.08 | 10 |
| Body Surface Area (m2) | 0.007 | 0.025 | 0.15 | 0.05 | 0.02 | 0.5 |
| Km factor | 3 | 6 | 12 | 8 | 5 | 20 |
| Animal A (mg/kg) = Animal B (mg/kg) multiplied by | Animal B Km |
| Animal A Km |
For example, to modify the dose of Compound A used for a mouse (20 mg/kg) to a dose based on the BSA for a rat, multiply 20 mg/kg by the Km factor for a mouse and then divide by the Km factor for a rat. This calculation results in a rat equivalent dose for Compound A of 10 mg/kg.
[2] Duran GE, et al. Mol Cancer Ther. Mechanisms of resistance to cabazitaxel.
| Related Products |
|---|
| C24-Ceramide
C24 Ceramide (Lignoceric ceramide) is a naturally occurring ceramide and an integral part of the sphingomyelin cycle. C24 Ceramide (Lignoceric ceramide) stimulates the proliferation of basophilic erythroblasts, suggesting a possible role in erythrocyte maturation. |
| RNA-protein crosslinking (sulfhydryl addition) agent
RNA-protein crosslinking (sulfhydryl addition) agent is a thiolating agent that is useful in cross-linking proteins. Thiolan-2-imine hydrochloride is a reagent for the introduction of sulphydryl groups into oligosaccharides derived from asparagine-linked glycans. |
| Izeltabart
Izeltabart is a high-affinity humanized antibody targeting ADAM9. Izeltabart can be used as ADC Antibody for site-specific conjugation with Maytansinoid-based DM21-C to synthesize IMGC936, an Antibody-drug Conjugate with strong anti-cancer activity. IMGC936 exhibits cytotoxicity against ADAM9-positive human tumor cell lines and potent antitumor activity in xenograft tumor models. |
| Cofetuzumab
Cofetuzumab (PF-06523435) is a humanized IgG1-κ monoclonal antibody targeting PTK7. |
| Crizanlizumab
Crizanlizumab is an anti-P-selectin monoclonal antibody. Crizanlizumab binds to P-selectin and blocks its interaction with P-selectin glycoprotein ligand 1 (PSGL-1). Crizanlizumab prevents vaso-occlusive crises (VOCs). |


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2023 AbMole BioScience. All Rights Reserved.
