All AbMole products are for research use only, cannot be used for human consumption.
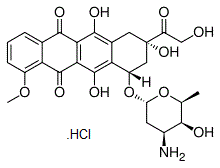
Doxorubicin (Adriamycin) HCl is an antineoplastic agent used in the treatment of a wide range of cancers, including hematological malignancies, many types of carcinoma, and soft tissue sarcomas. Doxorubicin interacts with DNA by intercalation and inhibition of macromolecular biosynthesis. Doxorubicin stabilizes the topoisomerase II complex after it has broken the DNA chain for replication, preventing the DNA double helix from being resealed and thereby stopping the process of replication. In studies with isolated nuclei, adriamycin was also a more potent inhibitor of DNA synthesis than RNA synthesis. However, with intact cells, adriamycin inhibited both DNA and RNA synthesis to about the same extent. The inhibition produced by adriamycin on RNA synthesis in intact cells was greater than that observed in the cell-free systems. Adriamycin inhibited protein synthesis in a cell-free system consisting of polyribosomes, transfer RNA, and enzymes but did not inhibit protein synthesis in intact cells. Combination therapy experiments with sirolimus (rapamycin) and doxorubicin have shown promise in treating Akt-positive lymphomas in mice.

Clin Transl Med. 2025 May 05;15(5):e70327.
Targeting capacity, safety and efficacy of engineered extracellular vesicles delivered by transdermal microneedles to treat plasmacytoma in mice
Doxorubicin HCL purchased from AbMole

Cancer Med. 2025 Apr 14;14(8):e70785.
Blockade of Exosome Release Sensitizes Breast Cancer to Doxorubicin via Inhibiting Angiogenesis
Doxorubicin HCL purchased from AbMole

Cancer Res. 2024 May 8.
HDAC Inhibition Increases CXCL12 Secretion to Recruit Natural Killer Cells in Peripheral T Cell Lymphoma | Cancer Research | American Association for Cancer Research
Doxorubicin HCL purchased from AbMole

ACS Appl Mater Interfaces. 2024 Feb;16(6):6868-6878.
Doxorubicin-Loaded Microalgal Delivery System for Combined Chemotherapy and Enhanced Photodynamic Therapy of Osteosarcoma
Doxorubicin HCL purchased from AbMole

Phytomedicine. 2024 Jan;126:155395.
Bufalin suppresses hepatocellular carcinogenesis by targeting M2 macrophage-governed Wnt1/β-catenin signaling
Doxorubicin HCL purchased from AbMole

ACS Appl Bio Mater. 2024 Sep 06.
Doxorubicin-Loaded Ultrasmall Gold Nanoparticles (1.5 nm) for Brain Tumor Therapy and Assessment of Their Biodistribution
Doxorubicin HCL purchased from AbMole

Eng. Regen. 2024 Mar.
Microalgae-based drug delivery system for tumor microenvironment photo-modulating and synergistic chemo-photodynamic therapy of osteosarcoma
Doxorubicin HCL purchased from AbMole

Elife. 2021 Jun 28;10:e65150.
Resistance to different anthracycline chemotherapeutics elicits distinct and actionable primary metabolic dependencies in breast cancer
Doxorubicin HCL purchased from AbMole

Mol Med Rep. 2021 Mar;23(3):219.
Shengxian decoction decreases doxorubicin‑induced cardiac apoptosis by regulating the TREM1/NF‑κB signaling pathway
Doxorubicin HCL purchased from AbMole

2020 Aug.
Blockage of AMPK-ULK1 pathway mediated autophagy promotes cell apoptosis to increase doxorubicin sensitivity in breast cancer (BC) cells: an in vitro study
Doxorubicin HCL purchased from AbMole

PLoS One. 2017 Jul 13;12(7):e0181340.
Development and characterization of a human three-dimensional chondrosarcoma culture for in vitro drug testing
Doxorubicin HCL purchased from AbMole

Biomaterials. 2012 Jun;4345-52.
Antitumor efficacy following the intracellular and interstitial release of liposomal doxorubicin
Doxorubicin HCL purchased from AbMole
| Cell Experiment | |
|---|---|
| Cell lines | Human choriocarcinoma cell line |
| Preparation method | Briefly, cells were plated on 96-well plates (10,000 cells/well) and a day after (cell culture 80% confluent) were exposed to doxorubicin (0–8 mM), L-DOX (0–8 mM) or PL-DOX (0–200 mM) in non-supplemented serum-free growth medium for 4 h. After exposure, the cells were washed and further incubated for 20 h. |
| Concentrations | 0–8 µM |
| Incubation time | 24 h |
| Animal Experiment | |
|---|---|
| Animal models | Male Sprague-Dawley rats |
| Formulation | |
| Dosages | 4 mg/kg |
| Administration | i.v. |
| Molecular Weight | 579.98 |
| Formula | C27H29NO11.HCl |
| CAS Number | 25316-40-9 |
| Solubility (25°C) | DMSO 45 mg/mL Water 30 mg/mL |
| Storage | 2-8°C, protect from light, dry, sealed |
| Related Animal Modeling Products |
|---|
| Sodium Thioglycolate
Sodium thioglycolate acts as reducing agent and is suitable for anaerobic and microaerophilic bacterial growth. Sodium thioglycolate is a commonly used reagent for bacteriological research to maintain reducing conditions in media. Thioglycolate can also protect enzymes against inactivation by maintaining protein thiol groups in the reduced state. Thioglycolate medium is frequently used in inflammation research to elicit a neutrophil and macrophage response in vivo. |
| Vancomycin-d10 2TFA salt
Vancomycin-d10 2TFA salt |
| Acetic acid-d4
Acetic acid-d4 |
| Myosin H Chain Fragment, mouse acetate
Myosin H Chain Fragment, mouse acetate salt is a fragment of the α-Myosin heavy chain peptide. Myosin H Chain Fragment can be used to induce experimental autoimmune myocarditis (EAM) mouse model. |
| Myosin H Chain Fragment, mouse
Myosin H Chain Fragment, mouse is a fragment of the α-Myosin heavy chain peptide. Myosin H Chain Fragment can be used to induce experimental autoimmune myocarditis (EAM) mouse model. |
All AbMole products are for research use only, cannot be used for human consumption or veterinary use. We do not provide products or services to individuals. Please comply with the intended use and do not use AbMole products for any other purpose.


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2024 AbMole BioScience. All Rights Reserved.
