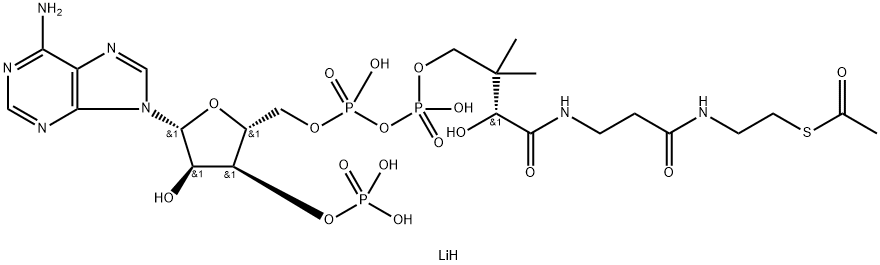
Acetyl-coenzyme A lithium regulates various cellular mechanisms by providing (sole donor) acetyl groups to target amino acid residues for post-translational acetylation reactions of proteins. Acetyl Coenzyme A lithium is also a key precursor of lipid synthesis.
| Molecular Weight | 809.57 |
| Formula | C23H38N7O17P3S |
| CAS Number | 32140-51-5 |
| Storage |
Powder -20°C 3 years ; 4°C 2 years In solvent -80°C 6 months ; -20°C 1 month |
| Species | Mouse | Rat | Rabbit | Guinea pig | Hamster | Dog |
| Weight (kg) | 0.02 | 0.15 | 1.8 | 0.4 | 0.08 | 10 |
| Body Surface Area (m2) | 0.007 | 0.025 | 0.15 | 0.05 | 0.02 | 0.5 |
| Km factor | 3 | 6 | 12 | 8 | 5 | 20 |
| Animal A (mg/kg) = Animal B (mg/kg) multiplied by | Animal B Km |
| Animal A Km |
For example, to modify the dose of Compound A used for a mouse (20 mg/kg) to a dose based on the BSA for a rat, multiply 20 mg/kg by the Km factor for a mouse and then divide by the Km factor for a rat. This calculation results in a rat equivalent dose for Compound A of 10 mg/kg.
| Related Metabolite/Endogenous Metabolite Products |
|---|
| Ursocholic acid
Ursocholic acid is a bile acid found predominantly in bile of mammals, which can be transformed into deoxycholic acid by the intestinal microflora in mice. |
| 5β-Cholanic acid
5β-Cholanic acid can be used for 5β-Cholanic acid derivatives synthesis. |
| D-Thyroxine
D-Thyroxine is a thyroid hormone that can inhibit TSH secretion. D-Thyroxine can be used for the research of hypercholesterolemia. |
| Allolithocholic acid
Allolithocholic acid is a steroid acid could found in normal serum and feces. Allolithocholic acid facilitates excretion, absorption, and transport of fats and sterols in the intestine and liver. |
| Biliverdin
Biliverdin is the product of the oxidation of bilirubin. Biliverdin can be synthesized via a non-protoporphyrin pathway in Corynebacterium glutamicum. |


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2023 AbMole BioScience. All Rights Reserved.
