| Product Name: | Fezagepras sodium |
| Catalog Number: | M28051 |
| CAS Number: | 1254472-97-3 |
Physical and Chemical Properties
| Formula: | C13H17NaO2 |
| Molecular Weight: | 228.26 |
| Form: | Solid |
| Solubility: | Water ≥ 100 mg/mL; DMSO ≥ 64 mg/mL |
| Storage: | 4°C, dry, sealed |
| Chemical Structure: | 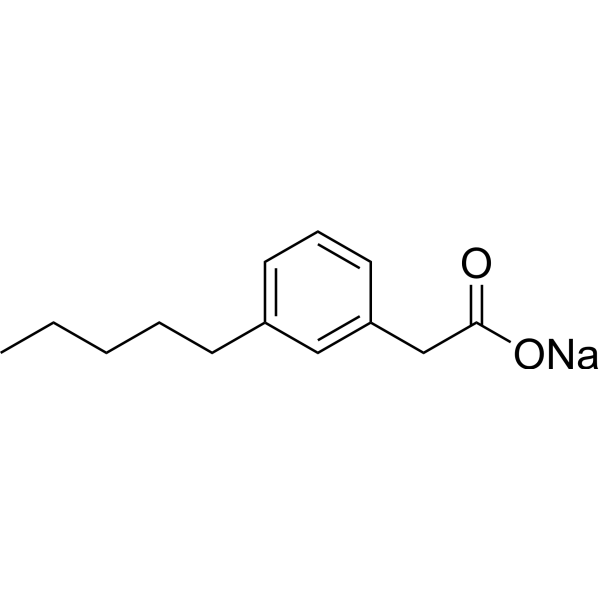 |
Analytical Data
| QC Standard: | ≥99.0% |
| H-NMR: | Consistent with structure |
Product Information
| Description: | Fezagepras (Setogepram) sodium acts as an orally active agonist for GPR40 and as an antagonist or inverse agonist for GPR84. Fezagepras sodium decreases renal, liver and pancreatic fibrosis. Fezagepras sodium exerts anti-fibrotic, anti-inflammatory and anti-proliferative actions. |
| Stability and Solubility Advice: | Information concerning product stability, particularly in solution, has rarely been reported and in most cases we can only offer a general guide. We recommend that stock solutions, once prepared, are stored aliquoted in tightly sealed vials and used within 1 month. Avoid repeated freeze and thaw cycles. Storage conditions for some special products should refer to their storage details. |
