| Product Name: | Ladarixin sodium |
| Catalog Number: | M30746 |
| CAS Number: | 865625-56-5 |
Physical and Chemical Properties
| Formula: | C11H11F3NNaO6S2 |
| Molecular Weight: | 397.32 |
| Form: | Solid |
| Solubility: | DMSO 100 mg/mL (ultrasonic) |
| Storage: | 4°C, dry, sealed |
| Chemical Structure: | 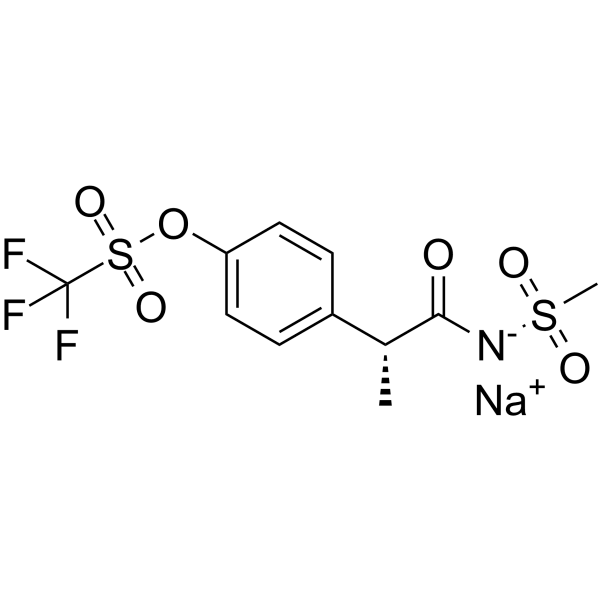 |
Analytical Data
| QC Standard: | ≥99.0% |
| H-NMR: | Consistent with structure |
Product Information
| Description: | Ladarixin sodium (DF 2156A) is an orally active, allosteric non-competitive and dual CXCR1 and CXCR2 antagonist. Ladarixin sodium can be used for the research of COPD and asthma. |
| Stability and Solubility Advice: | Information concerning product stability, particularly in solution, has rarely been reported and in most cases we can only offer a general guide. We recommend that stock solutions, once prepared, are stored aliquoted in tightly sealed vials and used within 1 month. Avoid repeated freeze and thaw cycles. Storage conditions for some special products should refer to their storage details. |
