| Product Name: | Danofloxacin |
| Catalog Number: | M27814 |
| CAS Number: | 112398-08-0 |
Physical and Chemical Properties
| Formula: | C19H20FN3O3 |
| Molecular Weight: | 357.38 |
| Storage: | Please store the product under the recommended conditions in the Certificate of Analysis. |
| Chemical Structure: | 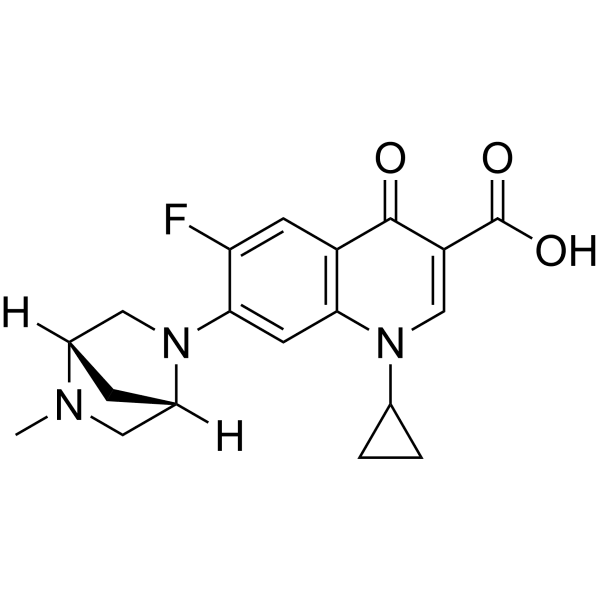 |
Analytical Data
| QC Standard: | ≥99.0% |
| H-NMR: | Consistent with structure |
Product Information
| Description: | Danofloxacin is a third generation fluoroquinolone and orally active antimicrobial agent. Danofloxacin shows a broad spectrum of activity against most Gram-negative and Gram-positive bacteria, mycoplasma and chlamydia species, and plays an antimicrobial role by inhibition of bacterial DNA-gyrase. Danofloxacinh has the potential for respiratory diseases in cattle, swine, and chickens treatment. |
| Stability and Solubility Advice: | Information concerning product stability, particularly in solution, has rarely been reported and in most cases we can only offer a general guide. We recommend that stock solutions, once prepared, are stored aliquoted in tightly sealed vials and used within 1 month. Avoid repeated freeze and thaw cycles. Storage conditions for some special products should refer to their storage details. |
