| Description: |
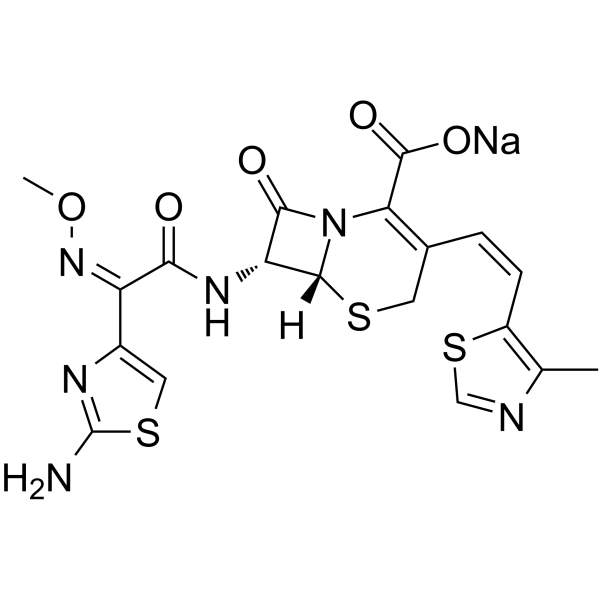
Cefditoren sodium (ME 1206) is a broad-spectrum, third-generation, oral cephalosporin antibacterial with enhanced stability against many common β lactamases. Cefditoren sodium has activity against Gram-negative organisms and Gram-positive organisms. Cefditoren sodium can be used in the research of infection diseases such as acute exacerbations of chronic bronchitis, community-acquired pneumonia (CAP), streptococcal pharyngitis/tonsillitis, or uncomplicated skin and skin structure infections. |
| Stability and Solubility Advice: |
Information concerning product stability, particularly in solution, has rarely been reported and in most cases we can only offer a general guide.
We recommend that stock solutions, once prepared, are stored aliquoted in tightly sealed vials and used within 1 month. Avoid repeated freeze and thaw cycles. Storage conditions for some special products should refer to their storage details. |