PD 0332991 is an orally active, highly selective inhibitor of the cyclin D kinases (CDK)4 and CDK6 with ability to block retinoblastoma (Rb) phosphorylation in the low nanomolar range. PD-0332991 selectively inhibits cyclin-dependent kinases (particularly Cdk4/cyclin D1 kinase), which may inhibit retinoblastoma (Rb) protein phosphorylation; inhibition of Rb phosphorylation prevents Rb-positive tumor cells from entering the S phase of the cell cycle (arrest in the G1 phase), resulting in suppression of DNA replication and decreased tumor cell proliferation. PD 0332991 was synergistic with tamoxifen and trastuzumab in ER+ and HER2-amplified cell lines, respectively. PD 0332991 enhanced sensitivity to tamoxifen in cell lines with conditioned resistance to ER blockade. Therapeutic doses of PD 0332991 cause elimination of phospho-Rb and the proliferative marker Ki-67 in tumor tissue and down-regulation of genes under the transcriptional control of E2F. The results indicate that inhibition of Cdk4/6 alone is sufficient to cause tumor regression and a net reduction in tumor burden in some tumors.

NPJ Breast Cancer. 2022 Jan;8(1):1.
Effects of neoadjuvant trastuzumab, pertuzumab and palbociclib on Ki67 in HER2 and ER-positive breast cancer
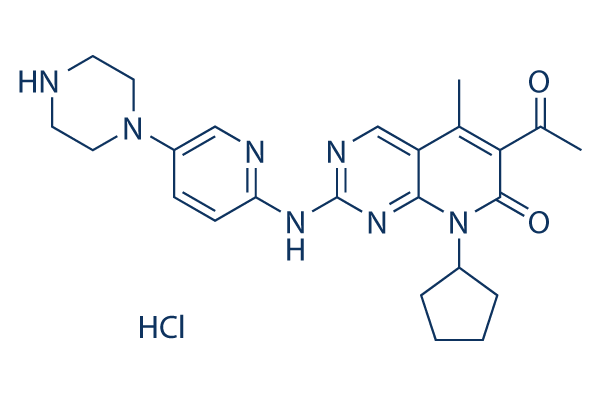
PD 0332991 HCL purchased from AbMole

BMC Biol. 2021 May 20;19(1):108.
Very long intergenic non-coding (vlinc) RNAs directly regulate multiple genes in cis and trans
PD 0332991 HCL purchased from AbMole

Journal of Molecular Structure. 2014 Jan 6;Pages 209-215.
A theoretical study of the structure and protonation of Palbociclib (PD 0332991)
PD 0332991 HCL purchased from AbMole
| Cell Experiment | |
|---|---|
| Cell lines | HCT116, SW480, Lovo and LS174T cells |
| Preparation method | Cell proliferation and colony formation assays Cells were seeded in a 96-well plate at 2000–6000 cells/well, incubated overnight at 37°C to allow adhesion, and then treated with inhibitors for 72 hours. Cell proliferation was determined using MTS solution (Promega), and formal assessment for synergy performed per the Chou Talalay method [64, 65] using CompuSyn (ComboSyn, Inc, Paramus, NJ). For colony formation assay, cells were seeded in a 6-well plate at 8000– 80,000 cells/well, incubated overnight at 37°C to allow adhesion, and then treated with inhibitors for 2–3 weeks. Cell colonies were fixed with ice-cold methanol and stained with 1% crystal violet. The density of colonies over the plate area was quantified by ImageJ (NIH) [66]. For detection of exposed phosphatidylserine residues reflective of apoptosis, Annexin V-FITC apoptosis assay kit (BD Biosciences) was used. Cells were seeded at 500,000 cells/ well in 6-well plates, incubated overnight at 37°C to allow adhesion, and then treated with inhibitors for 72 hours. Cells were washed and stained with annexin V-FITC and propidium iodide, and flow cytometry was performed using the Gallios flow cytometer (Beckman Coulter) and analyzed with Kaluza flow analysis software (Beckman Coulter). To measure cell senescence, treated cells were stained for senescence-associated beta-galactosidase (Chemicon). Cells were seeded at 500,000 cells/well in 6-well plates, incubated overnight at 37°C to allow adhesion, and then treated with inhibitors for 72 hours. Cells were washed with PBS, fixed with glutaraldehyde and methanol, washed twice more, and then incubated overnight in the dark at 37°C under ambient atmospheric conditions with X-Gal. Subsequently, cells were washed, and 10 high-powered light microscopy images of each well were captured, and cells with blue staining were manually counted. |
| Concentrations | 0~400nM |
| Incubation time | 72 h |
| Animal Experiment | |
|---|---|
| Animal models | Primary human-tumor xenograft models in NU/J6-week old female mice |
| Formulation | PBS |
| Dosages | 100 mg/kg per os daily for 21 days |
| Administration | oral gavage |
| Molecular Weight | 483.99 |
| Formula | C24H29N7O2.HCl |
| CAS Number | 827022-32-2 |
| Solubility (25°C) | DMSO 4 mg/mL warmed Water 30 mg/mL |
| Storage |
Powder -20°C 3 years ; 4°C 2 years In solvent -80°C 6 months ; -20°C 1 month |
| Species | Mouse | Rat | Rabbit | Guinea pig | Hamster | Dog |
| Weight (kg) | 0.02 | 0.15 | 1.8 | 0.4 | 0.08 | 10 |
| Body Surface Area (m2) | 0.007 | 0.025 | 0.15 | 0.05 | 0.02 | 0.5 |
| Km factor | 3 | 6 | 12 | 8 | 5 | 20 |
| Animal A (mg/kg) = Animal B (mg/kg) multiplied by | Animal B Km |
| Animal A Km |
For example, to modify the dose of Compound A used for a mouse (20 mg/kg) to a dose based on the BSA for a rat, multiply 20 mg/kg by the Km factor for a mouse and then divide by the Km factor for a rat. This calculation results in a rat equivalent dose for Compound A of 10 mg/kg.
| Related CDK Products |
|---|
| PF-07224826
PF-07224826 is a CDK2/4/6 inhibitor. |
| INCB123667
INCB123667 is a CDK2 inhibitor. |
| Senexin B
Senexin B is a potent, orally active CDK8/19 inhibitor with Kd values of 140 nM and 80 nM for CDK8 and CDK19, respectively. |
| Q901
Q901 is a CDK7 inhibitor for tumor-related studies. |
| NUV-422
NUV-422 is a CDK2/4/6 inhibitor that can be used in studies related to malignant gliomas. |


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2023 AbMole BioScience. All Rights Reserved.
