Niraparib tosylate inhibits PARP1/PARP2 with IC50 of 3.8 nM/2.1 nM respectively. MK-4827 (Niraparib) inhibits PARP activity with EC50=4 nM and EC90=45 nM in a whole cell assay. MK-4827 inhibits proliferation of cancer cells with mutant BRCA-1 and BRCA-2 with CC50 in the 10−100 nM range.
MK-4827 is well tolerated in vivo and demonstrates efficacy as a single agent in a xenograft model of BRCA-1 deficient cancer. MK-4827 enhances radiation response of p53 mutant Calu-6 tumor in both cases, with the single daily dose of 50 mg/kg being more effective than 25 mg/kg given twice daily.
| Cell Experiment | |
|---|---|
| Cell lines | V-C8 cells |
| Preparation method | V-C8 (BRCA2-negative) Chinese hamster cells are treated with the PARP inhibitor MK-4827 for 24 h, washed and incubated in drug-free medium for 5-7 days until colonies formed. |
| Concentrations | 50 nM |
| Incubation time | 24 h |
| Animal Experiment | |
|---|---|
| Animal models | Female nude mice (Ncr Nu/Nu) |
| Formulation | Suspended in 0.5% Methocel in deionized water |
| Dosages | 25 or 50 mg/kg |
| Administration | p.o. |
| Molecular Weight | 492.59 |
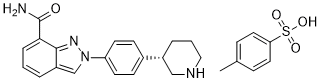
| Formula | C19H20N4O.C7H8O3S |
| CAS Number | 1038915-73-9 |
| Solubility (25°C) | DMSO: ≥ 60 mg/mL |
| Storage |
Powder -20°C 3 years ; 4°C 2 years In solvent -80°C 6 months ; -20°C 1 month |
| Species | Mouse | Rat | Rabbit | Guinea pig | Hamster | Dog |
| Weight (kg) | 0.02 | 0.15 | 1.8 | 0.4 | 0.08 | 10 |
| Body Surface Area (m2) | 0.007 | 0.025 | 0.15 | 0.05 | 0.02 | 0.5 |
| Km factor | 3 | 6 | 12 | 8 | 5 | 20 |
| Animal A (mg/kg) = Animal B (mg/kg) multiplied by | Animal B Km |
| Animal A Km |
For example, to modify the dose of Compound A used for a mouse (20 mg/kg) to a dose based on the BSA for a rat, multiply 20 mg/kg by the Km factor for a mouse and then divide by the Km factor for a rat. This calculation results in a rat equivalent dose for Compound A of 10 mg/kg.
| Related PARP Products |
|---|
| DSB-1522
DSB-1522 is a PARP1 inhibitor that can be used in tumor-related studies. |
| DM-5167
DM-5167 is a PARP1 inhibitor that can be used in studies related to triple negative breast cancer. |
| NMS-03305293
NMS-03305293 is a PARP inhibitor with high selectivity for PARP1 isoforms and low DNA capture effect, which specifically kills BRCA mutated tumor cells. In addition, NMS-03305293 can penetrate the blood-brain barrier and can be used in studies related to CNS tumors and brain metastatic tumors. |
| SNV1521
SNV1521 is a potential best-in-class (BIC), highly selective and CNS-permeable PARP1 inhibitor. |
| Basroparib
Basroparib is an orally active selective inhibitor of end-anchor polymerase (TNKS) and an inhibitor of ribose polymerase (PARP) with antitumor activity. |


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2023 AbMole BioScience. All Rights Reserved.
