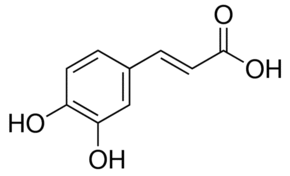
Caffeic acid inhibited 5-lipoxygenase in a non-competitive manner. Caffeic acid and its methyl ester did not inhibit prostaglandin synthase activity at all, at least up to 5 X 10(-4) M, but rather stimulate at higher doses.Caffeic acid has been shown to inhibit arachidonic acid metabolism in platelets at high doses.It has been observed to stimulate prostaglandin synthesis at high doses. Caffeic acid is an inhibitor of GST, ODC, Tyk and Xanthine Oxidase.Caffeic acid inhibits the synthesis of leukotrienes that are involved in immunoregulation, inflammation and allergy.Caffeic acid inhibits Cu2+-induced LDL oxidation.
| Molecular Weight | 180.16 |
| Formula | C9H8O4 |
| CAS Number | 331-39-5 |
| Solubility (25°C) | DMSO 40mg/mL Ethanol 25mg/mL |
| Storage |
Powder -20°C 3 years ; 4°C 2 years In solvent -80°C 6 months ; -20°C 1 month |
| Species | Mouse | Rat | Rabbit | Guinea pig | Hamster | Dog |
| Weight (kg) | 0.02 | 0.15 | 1.8 | 0.4 | 0.08 | 10 |
| Body Surface Area (m2) | 0.007 | 0.025 | 0.15 | 0.05 | 0.02 | 0.5 |
| Km factor | 3 | 6 | 12 | 8 | 5 | 20 |
| Animal A (mg/kg) = Animal B (mg/kg) multiplied by | Animal B Km |
| Animal A Km |
For example, to modify the dose of Compound A used for a mouse (20 mg/kg) to a dose based on the BSA for a rat, multiply 20 mg/kg by the Km factor for a mouse and then divide by the Km factor for a rat. This calculation results in a rat equivalent dose for Compound A of 10 mg/kg.
| Related HDAC Products |
|---|
| Ac-Arg-Gly-Lys(Ac)-AMC
Ac-Arg-Gly-Lys(Ac)-AMC is a substrate for HDAC. |
| Chlamydocin
Chlamydocin, a fungal metabolite, is a highly potent HDAC inhibitor, with an IC50 of 1.3 nM. |
| HDAC-IN-30
HDAC-IN-30 is a novel multi-target HDAC inhibitor, including HDAC1 (IC50=13.4 nM),HDAC2 (IC50=28.0 nM), HDAC3 (IC50=9.18 nM), HDAC6 (IC50=42.7 nM), HDAC8 (IC50=131 nM). |
| Ac-Arg-Gly-Lys(Ac)-AMC acetate
Ac-Arg-Gly-Lys(Ac)-AMC acetate is a substrate for histone deacetylase (HDAC) and can be used in a novel fluorescent assay for HDAC activity. |
| JPS014 TFA
JPS014 TFA is a benzamide-based Von Hippel-Lindau (VHL) E3-ligase proteolysis targeting chimeras (PROTAC). |


Products are for research use only. Not for human use. We do not sell to patients.
© Copyright 2010-2023 AbMole BioScience. All Rights Reserved.
